Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Peptide Denies Cell Entry To Ebola Virus
With a newly designed peptide, scientists have developed a way to inhibit the deadly virus
by Stu Borman
April 18, 2011
| A version of this story appeared in
Volume 89, Issue 16

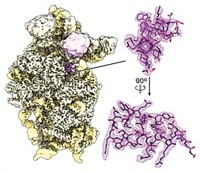
A novel approach to fight Ebola virus uses a designed peptide to inhibit the deadly virus’ entry into host cells. Ebola infection causes a rapidly progressing condition with internal and external bleeding and high fever. The disease has a high mortality rate, and there are currently no approved vaccines or therapies. Infection begins when a viral surface glycoprotein catalyzes fusion between the virus’s membrane and the membrane of endosomes in host cells. C-peptides inhibit similar fusion reactions involving viruses such as HIV and SARS, but they have been ineffective against Ebola. Kartik Chandran and Jonathan R. Lai of Albert Einstein College of Medicine and coworkers have now boosted the activity of Ebola C-peptide by conjugating it to an endosome-targeting sequence (J. Biol. Chem., DOI: 10.1074/jbc.m110.207084). They show that the modified peptide has potent in vitro anti-Ebola activity, and they determined its mechanism of action. The approach “is amenable to targeting other viruses whose fusion intermediates are exposed in the endocytic pathway and will provide a useful suite of research tools to probe intermediates in the process of viral entry,” Lai says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter