Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Unveiling Drug Candidates
ACS Meeting News: Medicinal chemists reveal potential drugs for HIV, Alzheimer’s, diabetes, and irritable bowel syndrome
by Carmen Drahl
April 18, 2011
| A version of this story appeared in
Volume 89, Issue 16

The Anaheim Convention Center last month hosted the latest installment of an American Chemical Society national meeting tradition—the first public disclosure of the structures of five experimental medicines. The event highlighted compounds that had reached human clinical trials, as well as the chemistry strategies that led researchers to each molecule.
Robert J. DeVita, a director of medicinal chemistry at Merck’s Rahway, N.J., discovery chemistry site, presided over the session, held in the Division of Medicinal Chemistry and entitled “First Time Disclosure of Clinical Candidates.” The symposium was organized by Albert J. Robichaud, vice president of chemistry at Danish drugmaker H. Lundbeck, and was sponsored by Gilead Sciences.
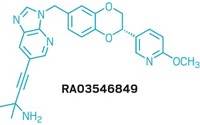
Starting off the symposium, Robert L. Hudkins, distinguished scientist at Cephalon in Frazer, Pa., disclosed the structure of CEP-26401 (irdabisant), a histamine H3 receptor blocker for treating cognitive deficits in multiple diseases, including Alzheimer’s disease.
The histamine H3 receptor regulates the release of multiple neurotransmitters that play a role in memory, attention, and cognition. Blocking the H3 receptor would promote release of those neurotransmitters, in effect aiding several cognition-related processes with one pill, Hudkins explained.
The Cephalon team’s quest drew on the history of failures in the H3 area. “Some compounds from the industry have moved into clinical trials, but many have failed,” Hudkins said. No drug targeting this histamine receptor has yet reached the market, he added. Several of the issues that led to earlier failures, such as cardiovascular side effects, could be tied to the molecules’ lipophilicity. Yet Cephalon couldn’t eliminate lipophilicity entirely, because some lipophilicity is necessary for getting a drug into the brain.
By placing lipophilicity among its top priorities, the team eventually developed irdabisant, a drug candidate taken in pill form that acts as an inverse agonist at the histamine H3 receptor. The molecule not only blocks the receptor’s response to histamine but also blocks the receptor’s low-level baseline activity, Hudkins explained.
In rats, irdabisant improved short-term memory, something that is thought to be impaired in Alzheimer’s disease. Phase I clinical trials for irdabisant, which evaluate safety and dosing, will finish this month, and Cephalon plans to advance irdabisant to Phase II trials. Cephalon is currently the object of a hostile takeover bid by Canada’s Valeant Pharmaceuticals, potentially clouding the future of Cephalon’s R&D projects (C&EN, April 4, page 16).
Following Hudkins’ disclosure, John F. Kadow, director of virology discovery chemistry at Bristol-Myers Squibb in Wallingford, Conn., told the story of BMS-663068, a potential treatment for the human immunodeficiency virus (HIV).
Over the past 30 years, HIV has become a manageable disease for many patients. But some patients don’t respond to available treatments. “A new drug with a new mechanism of action might be useful in a different combination,” which might help patients who are resistant to established drugs, Kadow said.

BMS targeted the earliest stage of viral attachment: the binding of the envelope protein gp120, a glycoprotein on the virus’s surface, to CD4, a glycoprotein on the surface of T cells. The envelope protein’s Achilles’ heel is a highly conserved small pocket where CD4 binds. BMS’s small-molecule drug candidates were found to bind in that small pocket, Kadow said.
BMS-663068 is the latest in a series of HIV attachment blockers that the company has brought to human clinical trials. Kadow and his colleagues optimized the screening hit BMS-216 to obtain BMS-378806. But when the compound was tested in humans, not enough of it reached the bloodstream to work against HIV. The team’s next effort, BMS-488043, lowered some patients’ HIV levels in clinical trials but had to be taken with high-fat meals because of low solubility.
BMS-663068 is a phosphate prodrug that arose from the team’s efforts to boost BMS-488043’s solubility and antiviral activity while maintaining its safety. The prodrug improved solubility, while replacing a methoxy group with a heterocycle improved activity.

In Phase IIa studies—a small-scale test of efficacy in HIV patients—BMS-663068 lowered viral RNA levels and increased counts of immune cells after eight days of treatment. A Phase IIb study, a larger efficacy test, is planned to start later this year, Kadow said.
After Kadow, Emma R. Parmee, executive director and head of discovery chemistry at Merck in West Point, Pa., disclosed the structure of MK-0893, which blocks the receptor for the hormone glucagon. The molecule is a potential treatment for type 2 diabetes.
When glucagon activates its receptor, it triggers glucose production in the liver, thus raising glucose levels. Type 2 diabetics “produce more glucagon than they should, both before and after meals,” Parmee said. As a result, these patients produce too much glucose, and their glucose production levels directly correlate with the severity of their diabetes, Parmee said.
“We looked at a lot of different chemical scaffolds” to find a glucagon receptor blocker that could be taken in pill form, Parmee said. The scaffolds Parmee discussed in Anaheim, Calif., were pyrazoles—which evolved from a urea—and benzimidazoles.

Although the pyrazoles failed to block the glucagon receptor as well as the benzimidazoles did, the Merck chemists prioritized pyrazoles because they had fewer metabolic drawbacks. To improve the pyrazoles’ ability to block the receptor, they added a methyl group at the benzylic position of their molecules. This strategy had not panned out with previous compound series, but the team attempted it anyway, a move that ultimately led them to MK-0893. “Don’t be afraid to try things you’ve tried before,” Parmee advised. “I was surprised how well it worked.”
In animal tests, MK-0893 was efficacious at lowering glucose production in the liver and had a favorable safety profile. According to the federal clinical trial registry ClinicalTrials.gov, several Phase II trials of the drug candidate in type 2 diabetics have been completed. MK-0893 does not appear in Merck’s Phase II pipeline in its most recent annual report.
Also at the Anaheim session, Alan J. Main, executive vice president of pharmaceutical research at Lexicon Pharmaceuticals in Princeton, N.J., described LX1031, an orally administered serotonin synthesis inhibitor for treating irritable bowel syndrome.
“People associate serotonin with the brain but the vast majority of it is produced in the gastrointestinal system,” Main told C&EN. Serotonin affects the GI tract in several ways, and studies relate high serotonin levels in the gut to GI disorders such as irritable bowel syndrome, which is marked by either diarrhea or constipation accompanied by abdominal pain.
Lexicon typically chooses targets after in-depth analysis of knockout mice from its library, which contains knockouts of more than 5,000 different enzymes or receptors. This study was no exception. The team noticed that a mouse lacking tryptophan hydroxylase, a key enzyme for serotonin biosynthesis, had reduced serotonin levels in the gut but not in the brain. From this data they went on to discover that two subtypes of the enzyme exist, one in the gut and one in the brain, implying that chemists might be able to target the subtype in the gut to treat irritable bowel syndrome.
Because X-ray crystal structures revealed that the two subtypes of tryptophan hydroxylase had similar active sites and binding pockets, the team opted to develop a serotonin synthesis inhibitor that stayed out of the brain and remained in the GI tract, Main said. Two strategies that served the team well were making molecules with molecular weights slightly above 550 and using highly polar functional groups such as an amino acid moiety to keep the compound from crossing into the brain. Both those strategies are evident in LX1031’s structure, he said.

To confirm that LX1031 stays out of the brain, the team has performed animal studies with radiolabeled versions of the molecule. Neurological side effects have not been seen in clinical studies, Main said.
LX1031 has completed Phase IIa clinical trials, which are an initial proof-of-concept test of efficacy, involving patients with nonconstipating irritable bowel syndrome, and the company plans to start larger Phase IIb clinical studies, Main said.

In the session’s final disclosure, South San Francisco-based Elan Pharmaceuticals principal scientist Gary G. Probst revealed the structure of ELND006, a molecule designed to treat Alzheimer’s disease by acting on the enzyme γ-secretase.
Elan is one of many companies eager to test whether halting production of amyloid-β, the peptide behind the plaques marring Alzheimer’s patients’ brains, can slow or stop the progression of the disease (C&EN, April 5, 2010, page 12). And γ-secretase is one of two key enzymes in amyloid-β production, Probst explained.
Selectivity was the name of the game in Elan’s medicinal chemistry program, Probst said. γ-Secretase is a membrane-bound protease that cleaves several peptide substrates besides amyloid-β, including the critical transcription factor Notch, which is involved in events such as cell-to-cell communication. “You want your inhibitor to tell γ-secretase to stop producing amyloid-β peptides but to continue to produce Notch,” Probst said. At the spring 2009 ACS national meeting in Salt Lake City, chemists at Bristol-Myers Squibb also emphasized the importance of this selectivity when disclosing their γ-secretase inhibitor BMS-708163 (C&EN, April 27, 2009, page 31).

During the discovery process, the Elan team switched from molecules with a caprolactam scaffold to molecules with a pyrazole scaffold. Pyrazoles had both better avoidance of Notch and improved penetration into the central nervous system. The team also added a trifluoromethyl group and a cyclopropyl group to enhance their molecules’ metabolic stability. These strategies eventually led the chemists to ELND006.

In humans, the molecule didn’t interfere with Notch and lowered amyloid-β levels by up to 50%, as measured from cerebrospinal fluid. But ELND006 had side effects in the liver that were unrelated to Notch, Probst said. Elan’s development program for ELND006 was halted in October 2010. But because the adverse effects appear to be unrelated to the molecule’s mechanism of action, Elan is continuing to study other γ-secretase inhibitors at earlier stages of its pipeline, with the goal of finding a drug candidate that can put the question of amyloid-β to the test.
Asked about the significance of the symposium, presider DeVita said that chemists attend the event largely for two reasons: “People want to see who’s innovating from a scientific standpoint, and people want to see what their competitors are working on.” But at the end of the day, “this symposium is about medicinal chemists’ creativity and problem-solving ability,” he added.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter