Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Extra Chlorophyll Helps Bacteria Photosynthesize
Eighth and previously unknown bacteriochlorophyll may be missing link to help scientists understand how bacteria turn sunlight into chemical energy
by Jyllian N. Kemsley
April 25, 2011
| A version of this story appeared in
Volume 89, Issue 17
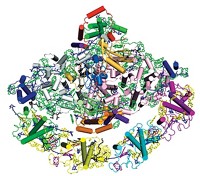
A key photosynthesis protein in certain bac teria contains eight bacteriochlorophyll molecules—one more than previously realized (Biochemistry, DOI: 10.1021/bi200239k). The additional compound is critical to understanding and modeling exactly how the bacteria turn sunlight into chemical energy. In green sulfur bacteria, the photosynthetic machinery is made up of an antenna complex that acts like a satellite dish to gather energy from sunlight, a reaction center that does electron-transfer chemistry, and the FMO protein (trimer shown) that acts like a wire to connect the two. Energy flows through seven bacteriochlorophyll compounds in FMO through a delocalized, quantum coherence mechanism, but researchers didn’t understand how energy got from the antenna complex into FMO. In new work, Robert E. Blankenship, Michael L. Gross, and colleagues at Washington University in St. Louis used mass spectrometry to demonstrate that a previously unidentified bacteriochlorophyll (shown in pink) likely provides the missing link. The compound probably resides on the FMO surface in an area that would interface with the antenna complex.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter