Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Impeding Cisplatin’s Power
Finding could guide changes that make anticancer drug more potent with fewer side effects
by Stu Borman
April 25, 2011
| A version of this story appeared in
Volume 89, Issue 17
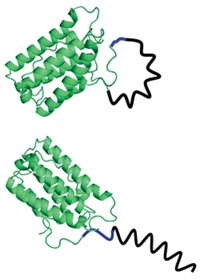
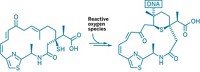
New findings on cellular interactions of the chemotherapeutic agent cisplatin suggest ways the drug might be redesigned to have better potency and fewer side effects. Cisplatin is an important chemotherapy for severe connective tissue, lung, ovarian, and lymphatic solid tumors. But it has serious adverse side effects, and its tendency to bind cytoplasmic proteins diverts it from its main job—binding and damaging genomic DNA in cancer cell nuclei, killing the cells. Now, researchers led by Pernilla Wittung-Stafshede and Magnus Wolf-Watz of Umeå University, in Sweden, report that cisplatin binds in solution to Atox1, a chaperone protein that helps provide copper to newly biosynthesized metalloproteins, and causes Atox1 to slowly unfold and degrade (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1012899108). They also show that cancer cells that express higher levels of Atox1 are more resistant to cisplatin. Spending its precious time messing around with Atox1 may keep some administered cisplatin from reaching DNA, suggesting that inhibiting the interaction might be a good way to make the drug safer and more effective.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter