Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Big Squeeze Clears Out Protein Garbarge
A mega cellular enzymatic machine undergoes a large conformational change to spit out its waste
by Sarah Everts
May 16, 2011
| A version of this story appeared in
Volume 89, Issue 20
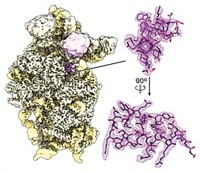
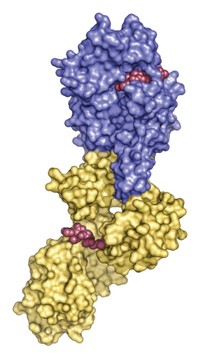
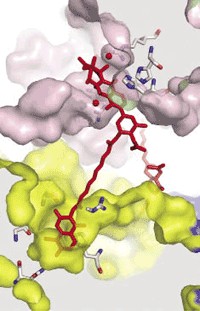
X-ray crystallography has revealed how a huge cellular enzymatic machine called ClpP—used by pathogenic microbes to break down proteins—spits out its waste: A large conformational change squeezes the protease’s cylinder, thereby creating large equatorial pores from which the refuse diffuses away. Researchers are particularly interested in ClpP’s structure because pathogenic bacteria such as Staphylococcus aureus use the enzyme to destroy signals that block infection. Scientists see ClpP as a possible target for antimicrobial drugs, and knowing how it operates is helpful for those creating inhibitors. The first suggestion of ClpP’s compacting conformation came last year from the University of Toronto’s Walid A. Houry and colleagues, who predicted it would feature equatorial exit pores (Structure, DOI: 10.1016/j.str.2010.04.008). Biochemists led by Stephan A. Sieber at Germany’s Technical University of Munich and Patrick Cramer of Ludwig Maximilian University, also located in Munich, now show that the conformational change flattens ClpP by 10 Å and produces 14 equatorial side pores that are each about 6 Å in diameter (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201100666). The new ClpP structure could help drug designers create molecules that act as wrenches in pathogen garbage compactors.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter