Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Supercapacitors Made From Super Carbon
Carbon material with a 3-D porous structure has exceptional surface area and electrical properties
by Mitch Jacoby
May 16, 2011
| A version of this story appeared in
Volume 89, Issue 20
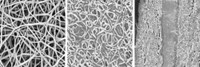
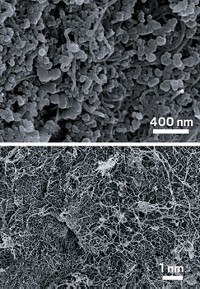
Simple chemical processing converts graphite oxide into a porous graphene-like carbon material with exceptional surface area and electrical properties, according to a team led by researchers at the University of Texas, Austin (Science, DOI: 10.1126/science.1200770). The development could lead to commercial quantities of this inexpensive form of carbon, which the team has shown can be fashioned into high-performance supercapacitors—devices typically made from high-surface-area carbon. Unlike batteries, which are designed to store a relatively large electrical charge and deliver energy slowly, supercapacitors deliver energy rapidly but in limited quantities. They are used in various types of electric vehicles and in consumer electronics. Yanwu Zhu, Rodney S. Ruoff, and coworkers used microwaves or heat to exfoliate graphite oxide and then treated the material with potassium hydroxide solution. The procedure formed a continuous 3-D porous network of mainly one-atom-thick carbon sheets with a surface area of some 3,100 m2/g. That value is far higher than the 1,000 to 2,000 m2/g typical of activated carbon and 500 m2/g higher than the theoretical value for graphene. Supercapacitors made from the material and subjected to standard test conditions have four times higher energy density and 10 times higher power density than today’s commercial activated-carbon supercapacitors, the team reports.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter