Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Cataracts Via Protein Interactions
Experimental, theoretical tools reveal a new route to the eye disease
by Stu Borman
January 17, 2011
| A version of this story appeared in
Volume 89, Issue 3

For some human cataracts caused by genetic mutation, the change in eye-lens opacity that results in the condition can be due to a protein interaction, researchers have found. Previously known mechanisms of cataract formation induced by genetic mutation involve problems with a single eye protein, such as damage, destabilization, or loss of solubility and consequent condensation of that one protein. The new study shows that cataracts also can arise from a mutation in the lens protein γ-crystallin that changes how that protein interacts with another lens protein, α-crystallin (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1014653107).
The findings—by associate professor of chemistry Jayanti Pande, an expert on chemical mechanisms of eye disease at the State University of New York, Albany, and coworkers—may not have immediate clinical significance. But the disclosure of a heretofore unknown class of cataract formation mechanism contributes to a deeper understanding of the way changes in protein interactions can cause cataracts and possibly other diseases as well.
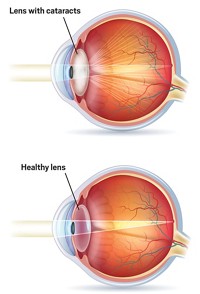
Cataract, which is cloudiness in the lens of the eye, is most commonly age-related but can also be caused by genetic mutation or by environmental factors such as ultraviolet light exposure and poor diet. In developed countries, the condition is treated surgically, but worldwide it remains a leading cause of blindness.
The new study focuses on a subtle mutation in γ-crystallin involving a single amino acid substitution of alanine for glutamate. The change leaves the protein’s overall structure intact but alters how it interacts with α-crystallin. Other cataract-related mutations affect individual eye proteins, such as truncation of the sequence of a single crystallin, making it insoluble.
The study uses experimental light-scattering and phase separation methods, in combination with free-energy and electrostatic modeling, to show that the glutamate-to-alanine modification boosts attractive, likely electrostatic, interactions between the smaller γ-crystallin and the larger α-crystallin. The enhanced attraction tends to cause aggregation, which increases light scattering by the proteins, leading to lens opacity.
These experimental findings confirm the results of an earlier, primarily theoretical study on cataract-related aggregation by theoretical physicist Giuseppe Foffi of EPFL, the Swiss Federal Polytechnic Institute of Lausanne; biological and chemical physicist George M. Thurston of Rochester Institute of Technology; soft matter physicist Peter Schurtenberger, now at Lund University, in Sweden; and coworkers (Phys. Rev. Lett., DOI: 10.1103/PhysRevLett.99.198103). They used molecular dynamics simulations and small-angle neutron scattering to predict that crystallin-like spheres of two different sizes mix evenly when they attract one another a little, tend to segregate when they do not attract each other, and aggregate into clumps when they attract one another a lot. In a new study, Foffi, EPFL postdoc Nicolas Dorsaz, and coworkers use thermodynamic perturbation theory to confirm yet again this same type of behavior by binary eye-lens protein mixtures (Soft Matter, DOI: 10.1039/c0sm00156b).
Dorsaz says the experimental findings by Pande and coworkers “are a major breakthrough in our understanding of possible mechanisms of eye-lens opacity in human cataract. For the first time, a mutation that does not alter the structure, stability, and phase diagram of γ-crystallin has been shown to destabilize, in a quite dramatic manner, mixtures of this protein and α-crystallin.
“Their experiment is proof of principle that the increase of light scattering observed in some forms of cataract could originate simply from a modification of the effective interaction between different crystallin proteins,” Dorsaz continues. “Their findings have the potential to bring together the chemistry, biology, and physics communities, which have been working on very different routes to cataract formation in the last years without too much interaction.”
J. Fielding Hejtmancik, a specialist in eye disease mechanisms at the National Institutes of Health’s National Eye Institute, says the Pande group’s finding “is, as far as I know, unique among described cataract mechanisms. It’s the first time I’ve seen an interaction with a different crystallin cause a cataract. Most mutations tend to destabilize a crystallin protein in a more radical fashion, such as by protein truncation or genetic frame shifts. This, on the other hand, is a pretty subtle change, and it’s an impressive piece of work to have been able to show this mechanism, which is very believable.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter