Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Knockout Pictures
X-ray structures of general anesthetics binding to an ion channel could aid search for new anesthetics
by Carmen Drahl
January 24, 2011
| A version of this story appeared in
Volume 89, Issue 4

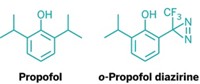
A close-up of an ion channel from blue-green algae reveals that two common anesthetics bind to it in roughly the same place (Nature, DOI: 10.1038/nature09647). This information could help researchers design new anesthetics. General anesthetics keep patients unconscious during surgeries, but it’s not clear how they work at the molecular level. Now, a team led by Pierre-Jean Corringer of France’s Pasteur Institute has determined the X-ray crystal structure of the algal channel, which resembles human acetylcholine and GABA receptors, with the anesthetics propofol and desflurane. Both molecules bind in a cavity in the membrane-spanning portion of the channel. Propofol binds at the mouth of the cavity whereas desflurane binds deeper inside. Mutating amino acids lining the binding sites affected sensitivity to the anesthetics. Ion-channel expert Sergei I. Sukharev of the University of Maryland praised the work, adding “it would be terrific if the authors introduced similar mutations into related mammalian receptors and tested them for sensitivity to propofol and desflurane.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter