Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Shotgun Approach To Drugs
Pacifichem News: Polypharmacology gives rise to drugs with multiple targets
by Celia Henry Arnaud
January 24, 2011
| A version of this story appeared in
Volume 89, Issue 4

The archetype for a drug is a single compound that interacts exclusively with a single target. As difficult as that goal is to achieve, drug discoverers still strive to reach it.
But experience suggests that many drugs hit multiple targets. Instead of trying to find a “magic bullet” that rifles its way to a particular target, perhaps researchers should be thinking in terms of “magic shotguns” that hit a combination of targets, Wes Kroeze, a research associate in Bryan Roth’s lab in the department of pharmacology at the University of North Carolina, Chapel Hill, suggested during a symposium held last month at the 6th International Chemical Congress of Pacific Basin Societies, or Pacifichem.
This tendency of drugs to hit multiple targets is not all bad. Drug designers can harness it to find new purposes for existing drugs, to predict and mitigate side effects, or to intentionally design drugs that interact with two or more targets.
All of these strategies fall under the umbrella of “polypharmacology.” Researchers from academia and the pharmaceutical industry gathered at Pacifichem to address pharmacology in its various guises.
Trying to treat disease by hitting multiple targets simultaneously is not new. The most straightforward way of doing this is with “cocktails” of different drugs mixed in a fixed combination, such as those used to treat HIV/AIDS. Another common example is the handful of pills some people must take to control hypertension.
But taking combinations of drugs with mismatched pharmacokinetic profiles can lead to complications and unintended drug-drug interactions, said Leslie J. Browne, president and chief executive officer of Senesco Technologies, in New Brunswick, N.J. In addition, it’s easier to formulate a single pill with a single multiple-target compound than a pill with multiple active ingredients. There’s only one compound to formulate, and it has a single pharmacokinetic profile. And producing a single compound costs less than producing and mixing multiple compounds.
Single compounds that hit multiple targets come in a progression of forms, said Bruce D. Hammock, an entomology professor at the University of California, Davis. At one end, two ligands that are selective for different targets are joined by a cleavable linker. When such a compound enters the body, the linker quickly releases the individual ligands. In a sense, this represents a single compound in name only.
Moving along the progression, the target-selective ligands become increasingly integrated, first via a stable covalent linker that tethers the two ligands and eventually toward a wholly integrated compound in which the two ligands are merged into a single entity that retains the selectivities of the individual ligands.
Industry and academic researchers are bringing polypharmacology approaches to a number of disease areas.
In the antibacterial arena, drugs that shut down multiple functions within bacteria can reduce the likelihood that pathogens evolve resistance mechanisms against those drugs, Arnaud LeTiran of Vertex Pharmaceuticals said. He described work to combat bacterial resistance with a compound that targets both gyrase and topoisomerase IV, ubiquitous enzymes that are both essential in the replication of DNA.
Existing antibiotics target either gyrase or topoisomerase IV by inhibiting the enzyme’s DNA-binding subunit. Vertex is working to build an antibiotic that instead targets the adenosine triphosphate (ATP)-binding sites of both enzymes, LeTiran said.
After screening a library of more than 25,000 compounds, Vertex scientists optimized benzimidazole ureas to effectively target both enzymes. Because such compounds inhibit the same ATP-binding sites targeted by the antibiotic novobiocin, they used an existing crystal structure of novobiocin bound with gyrase to design compounds with improved antibacterial activity.
“The availability of structural information was key to rapid improvement,” LeTiran said. Vertex scientists found that a coplanar substituent at the C-7 position of the benzimidazole core is important for targeting both gyrase and topoisomerase IV, he said.
LeTiran did not reveal which compound Vertex is moving forward with but did say that it is active against many gram-positive pathogens—including multi-drug-resistant ones—and has been effective in animal models of bacterial infections.
One of the biggest challenges for polypharmacology is designing drugs that selectively interact with only a handful of kinases involved in a particular disease. The large family of kinase enzymes catalyzes protein phosphorylation, one of the most common posttranslational modifications. A typical way to inhibit kinases is by blocking the ATP-binding site, but if a drug hits one kinase that way, it’s likely to hit them all, said Shawn P. Williams, a researcher at GlaxoSmithKline.
Lapatinib is an approved cancer drug that selectively targets two receptor tyrosine kinases, EGFR and ErbB-2, Williams said. These kinases are normally involved in the embryonic development of epithelium-derived organs such as the brain, heart, and lungs, but they are also overexpressed in a variety of cancers. In breast cancer, their overexpression correlates with poor prognosis. GSK scientists wanted to inhibit both of these kinases while minimizing inhibition of other kinases, Williams said.
The GSK team selected lapatinib using a mathematical approach. The algorithm, based on 22 selection criteria, calculates an index value that represents how much a compound’s measured behavior profile deviates from the target product profile.
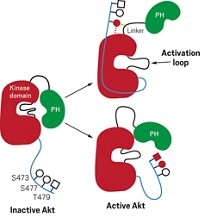
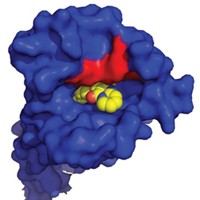
Lapatinib has unusual kinetic behavior, Williams reported. Its off-rate—which is a measure of how quickly a compound dissociates from its target once bound—is somewhere between the value expected for a covalent inhibitor and that expected for a compound with fast off-rate kinetics. They used X-ray crystallography to investigate whether this behavior is a property of the compound or of its effect on EGFR.
Some of the unusual behavior could be explained by the crystal structure, Williams said. When his team solved a crystal structure of lapatinib with EGFR, they observed that lapatinib triggers conformational changes in the enzyme that extend beyond the ATP-binding site. The drug’s large headgroup pushes up into a pocket, nudging a methionine out of the way. In this more compact, closed structure, the inactive enzyme collapses around the inhibitor in a closed, inert form. Movement of a helix in the enzyme pushes catalytic residues out of register. All of these things happen without the compound covalently binding to the kinase.
The project team concluded that the unusual kinetic behavior was due to both the molecule and its effect on the enzyme. “The conformational change and the unusual kinetic behavior was a direct consequence of lapatinib’s large headgroup,” Williams said. And it was the compound’s large headgroup that made lapatinib a good dual inhibitor. “The compounds that had the best dual-kinase and pan-kinase profiles all had large headgroups,” he said. The kinetic findings are true for ErbB-2 as well, but structures of that enzyme are harder to obtain, Williams said.
Yet another use of polypharmacology approaches could involve the design of new multitarget drugs that mitigate the side effects of existing drugs. Hammock’s group is working on compounds that inhibit both cyclooxygenase-2 (COX-2) and soluble epoxide hydrolase (sEH) as a way to treat inflammatory and neuropathic pain. Both of these enzymes are involved in the arachidonic acid cascade, which plays a role in inflammation, pain, and hypertension.
Administering sEH inhibitors and COX-2 inhibitors together decreases the production of prostaglandins, thereby causing both inflammation and pain to diminish, Hammock said. The hope is that integrating such inhibitors in a single compound will result in an anti-inflammatory effect with fewer of the cardiovascular side effects that are often seen when COX-2 inhibitors are administered by themselves.
Sung Hee Hwang, a postdoctoral researcher in Hammock’s group, used celecoxib as a starting point for finding dual COX-2/sEH inhibitors. He found a dual inhibitor that overlaps with celecoxib in models of COX-2 binding and also fits in the catalytic site of sEH, Hammock said. In diabetic rats, the dual COX-2/sEH inhibitor reduces neuropathic pain to a greater extent than the individual inhibitors administered alone or together, Hammock said.
In addition to assisting the discovery of new drugs, polypharmacology can help find new uses for old drugs or explain troublesome side effects, Kroeze said. “Many well-known drugs are highly promiscuous,” he said. For example, the schizophrenia drug clozapine affects 28 of the 71 targets Kroeze and his coworkers assayed it against, a number probably limited only by the number of assays. “Additional targets are likely to reveal additional hits,” he said.
Roth’s lab is gathering polypharmacology information into a database of known receptor-psychoactive drug pairs, Kroeze said. Users of the database, found online at pdsp.med.unc.edu, can mine the resource to learn what drugs hit a specific receptor or what receptors a drug interacts with. They intend to uncover such information for every target in the central nervous system, in part through their role of running the National Institute of Mental Health Drug Screening Program.
Understanding how drugs work in this new multitarget paradigm requires taking a systems-level approach to drug discovery, said Tatiana V. Khasanova, a scientist at Encinitas, Calif.-based GeneGo, which is now a Thomson Reuter company. She described how the company’s “knowledge base” of pharmacological interactions allows researchers to identify the various targets of a drug. The analysis can go in either direction, starting with a compound and finding targets or vice versa. For example, GeneGo’s application uncovered 268 indirect targets for the anticholesterol drug fenofibrate, which helps explain why the drug has anti-inflammatory activity and may explain its many side effects.
The days of the “magic bullet” are quickly disappearing, and researchers need to come to grips with the magic shotgun. With polypharmacology, maybe they’ll be able to sharpen the aim of their ammunition.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter