Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Surprising Superoxide
Catalysis: Novel mode of end‑on oxygen binding discovered in palladium complexes
by Stu Borman
January 24, 2011
| A version of this story appeared in
Volume 89, Issue 4

An international collaboration has discovered and characterized an unprecedented mode of binding of oxygen in Pd complexes with N-heterocyclic carbene (NHC) and/or PR3 (trialkyl- or triarylphosphine) ligands (J. Am. Chem. Soc., DOI: 10.1021/ja1103348).
The work is a fundamental advance in organometallic chemistry. But it could also have practical implications by aiding the development of new oxidation catalysts because Pd(NHC)(PR3) complexes are closely related to homogeneous catalysts for reactions that use molecular oxygen as an oxidant.
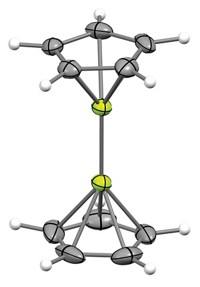
In a study of the kinetics and thermodynamics of O2 binding to Pd centers, Carl D. Hoff of the University of Miami and coworkers isolated and obtained the crystal structure of a new type of Pd(NHC)2 complex—a bis-superoxide complex with two end-on (η1) O2 ligands. In such complexes, only one of the two oxygen atoms in each superoxide group is linked to Pd. The researchers also found that this superoxide complex could undergo a hydrogen-atom transfer reaction to form a bis-hydroperoxide complex.
“The idea that a Pd(0) center reacts with two equivalents of O2 to form a stable bis-superoxide complex is remarkable,” says Shannon S. Stahl, a catalysis expert at the University of Wisconsin, Madison. That Hoff and coworkers “were able to get evidence for this—much less proceed to get a crystal structure and form the bis-hydroperoxide—there’s no way I would have believed this could happen.”
Stahl believes that the bis-superoxide also helps resolve a mechanistic controversy. He and his coworkers earlier prepared a Pd(NHC)2 side-on (η2) peroxide complex in which both oxygen atoms are bound to Pd and bonded to each other. On the basis of a computational study, they proposed that the side-on peroxide forms via an end-on (η1) intermediate. But this hypothesis conflicts with an alternative proposal that the reaction is a single-step process in which both oxygen atoms bind Pd simultaneously. The discovery of the end-on bis-superoxide provides the first experimental evidence for the end-on-intermediate hypothesis, Stahl says.
Hoff and coworkers find that the size of NHC and PR3 ligands in the Pd complexes appears to play a key role in determining the nature of oxygen binding. They propose that small ligands result in fast kinetics and the addition of a single O2 to form a side-on peroxide and that large ligands cause slow kinetics, providing extra time for the addition of both an initial end-on O2 and a second one, resulting in formation of the new end-on bis-superoxide.
Team member and inorganic chemistry professor Steven P. Nolan of the University of St. Andrews, in Scotland, says that the observation of novel end-on peroxide and hydroperoxide binding modes “may assist in designing ligand environments better suited for palladium-mediated oxidation catalysis.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter