Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catching Z Olefins
New catalysts solve selectivity problem and generate less stable Z olefins, rather than E olefins
by Bethany Halford
December 19, 2011
| A version of this story appeared in
Volume 89, Issue 51
COVER STORY
Catching Z Olefins
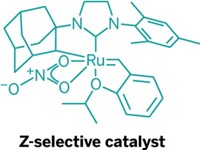
Scientists who had been losing sleep over the metathesis reaction’s preference to form E olefins were finally able to catch some Zs this year—Z olefins, that is. Using a new molybdenum catalyst, Boston College’s Amir H. Hoveyda, MIT’s Richard R. Schrock, and coworkers figured out a way to rig cross-metathesis reactions to preferentially produce the less stable, structurally cramped isomer with Z double bonds (C&EN, March 28, page 9; Nature, DOI: 10.1038/nature09957). Researchers led by Caltech’s Robert H. Grubbs were similarly able to selectively form Z olefins, but by using new ruthenium catalysts (C&EN, May 23, page 33; J. Am. Chem. Soc., DOI: 10.1021/ja202818v and 10.1021/ja210225e). In olefin metathesis chemistry, two carbon-carbon double bonds react to form a new alkene and an alkene by-product, usually ethylene. Through careful catalyst design, both groups were able to shift products toward the Z isomers. Grubbs and coworkers demonstrated the utility of the ruthenium catalysts by using them to make a pheromone-like molecule that is industrially relevant in insect pest control. Hoveyda and Schrock’s team used its molybdenum catalyst en route to preparing the immunostimulant KRN7000 and an antioxidant plasmalogen phospholipid. Expanding on their efforts to make Z olefins, Hoveyda and Schrock, working in collaboration with the University of Oxford’s Darren J. Dixon, also reported a tungsten catalyst that selectively forms Z alkenes in ring-closing metathesis reactions (C&EN, Nov. 7, page 11; Nature, DOI: 10.1038/nature10563). The researchers used the tungsten catalyst to stereoselectively synthesize the anticancer compound epothilone C and the antimicrobial compound nakadomarin A.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter