Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cross-Coupled Cyanamides
Utah chemists forge versatile N-C-N building blocks via a palladium-catalyzed cross-coupling reaction
by Stephen K. Ritter
December 19, 2011
| A version of this story appeared in
Volume 89, Issue 51
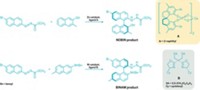
Palladium-catalyzed cross-couplings have become one of the most indispensable types of reactions in organic synthesis—they were the basis of the 2010 Nobel Prize in Chemistry. Chemists are still coming up with new applications for the method. In the latest example, Ryan M. Stolley, Wenxing Guo, and Janis Louie of the University of Utah report the first cross-couplings involving cyanamides (Org. Lett., DOI: 10.1021/ol203069p). Cyanamides are versatile N–C–N building blocks used to make guanidine-containing molecules, amidines, and a variety of heterocycles. These compounds find uses as antiviral and anticancer drugs, as well as organometallic ligands. Current methods to make cyanamides are sparse and tend to provide low yields, the researchers note. Louie’s team first screened precatalyst-ligand combinations and found that a palladium dibenzylideneacetone complex with the biphenyl phosphine ligand tert-butyl XPhos worked best. The Utah chemists then explored cyanamide cross-couplings with a range of aryl, heteroaryl, and vinyl partners. The reactions proceeded selectively under mild conditions within three hours in moderate to high yields, they report.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter