Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Spin On Stereocontrol
A molecular motor takes turns cranking out one enantiomer or another in an addition reaction
by Bethany Halford
December 19, 2011
| A version of this story appeared in
Volume 89, Issue 51

COVER STORY
New Spin On Stereocontrol
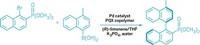
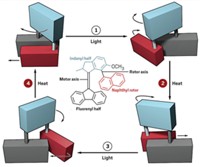
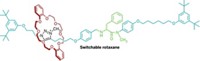
Challenging those who think molecular motor makers are just spinning their wheels, this year chemists in the Netherlands created a rotary motor catalyst molecule, driven by light and heat, that dynamically controls chirality in an asymmetric addition reaction (C&EN, Feb. 14, page 9; Science, DOI: 10.1126/science.1199844). The motor molecule, designed and built by the University of Groningen’s Ben L. Feringa and Jiaobing Wang, can make an R enantiomer, an S enantiomer, or a racemic mixture on demand, depending upon where the motor is in its rotary cycle. The catalytic motor consists of two arms that turn past one another in a unidirectional manner in four discrete steps. One arm is equipped with a 4-dimethylaminopyridine (DMAP) moiety, and the other arm features a thiourea group. When the arms are pointed away from each other, the catalyst generates a racemic mixture of products during the addition of 2-methoxythiophenol to cyclohexenone. But when light shines on the catalyst, its double-bond axle isomerizes, bringing the arms and their cooperative catalytic groups close to each other. Here, the thiourea group holds the cyclohexenone in place while the DMAP unit guides the thiophenol to stereoselectively form the addition product with S stereochemistry. Heating the system forces the arms to pass one another, which causes DMAP to send the thiophenol to the other side of the cyclohexenone and preferentially form the product with R stereochemistry. Light prompts the double bond to isomerize again and sends the arms back to their starting positions.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter