Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Meet The Ivyanes
Chemists create a new family of hydrocarbons in which cyclopropane rings hang off the sides of carbon chains
by Stephen K. Ritter
February 7, 2011
| A version of this story appeared in
Volume 89, Issue 6
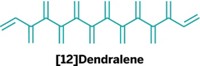
Chemists in Australia have given birth to a new family of hydrocarbons: the ivyanes. These oligocyclopropanes feature triangular rings hanging off the side of a carbon chain, a structure that resembles leaves on an ivy vine (Chem. Sci., DOI: 10.1039/c0sc00500b). In 2009, Michael S. Sherburn and coworkers at Australian National University reported the synthesis of dendralenes, a class of compounds in which each carbon in a chain is part of a branching alkene ideally situated to undergo cyclopropanation. Sherburn’s group has now used the Simmons-Smith reagent (diiodomethane and diethylzinc) to convert dendralenes with from three to eight alkenes to the corresponding ivyanes. The reactions are a chemical tour de force. For example, conversion of [8]dendralene to [8]ivyane involves formation of 16 C–C bonds. The shorter chain ivyanes are liquids at room temperature, whereas the [7] and [8] versions are solids. The compounds adopt chiral helical conformations in both solution and solid phases, a property that could be exploited in multiple applications. In addition, the unique ivyane framework prompted the team to try ring-opening reactions, which led to dimethyl-substituted chains with contiguous quaternary carbons, structures that are normally tough to make.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter