Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Race To The Pump
Biofuel technologies vie to provide a sustainable supply of transportation fuels
by Stephen K. Ritter
February 14, 2011
| A version of this story appeared in
Volume 89, Issue 7

Chemists, chemical engineers, and synthetic biologists have largely met the technical challenge of developing biofuels to supplement and then replace petroleum-derived transportation fuels in the coming decades. For biofuels to reach the U.S. market, however, these technologies have to fit into the existing transportation fuel infrastructure. Every major chemical and petrochemical firm has claimed a stake in the race to biofuel commercialization, as have dozens of start-up companies.
Biofuels have multiple starting points, including sugars, starches, vegetable oil, recycled paper and cardboard, and raw biomass, which can be processed by biological or chemical methods, or both. Whichever ones win, the competing technologies’ versatility ensures that companies will make money, and the country will gain energy security by eliminating dependence on imported oil, as well as climate security by reducing greenhouse gas emissions.

“We all know how to get from the beginning to the end and make biofuels—we’ve all done it,” says James A. Dumesic, a chemical engineer at the University of Wisconsin, Madison. “What you would like to do is put raw biomass in one end and get a ready-to-use fuel out the other end, using as few steps and engineering unit operations as possible. Now, we are to try to get the costs down so it can be affordable. The winning processes, whatever they will be, will need to be as light as possible on the capital investment in order to be practical. Everyone is looking to develop processes that can compete without subsidies.”
“Because the energy industry is so large, there is room for everybody to play, as long as you can meet the economics,” says Jay D. Keasling, a synthetic biologist at the University of California, Berkeley. “That is the great thing about this problem. Chemical technologies can be engineered to happen more quickly. It does take a long time to engineer the biology. But the beauty of biology is that it can work under dirtier conditions, and you can get the specific molecule you want under a range of conditions.”
Synthetic biology seemed to have the early edge in the race to the pump. But despite success in ethanol production, synthetic biology’s limitations—the primary products are alcohols, not alkanes typical of transportation fuels, and fermentation processes are slow—have stalled progress. That has created an opening for chemical technologies.
“Chemical approaches offer plenty of advantages,” says Mark Mascal, a chemistry professor at UC Davis whose group is working on several biofuel projects. “Generally, if you have an inexpensive catalyst and a fast method, a chemical approach can be more cost-effective and doesn’t take a few days or a week the way most fermentation processes do,” he notes. “A consistent feedstock isn’t needed as is the case with microbes in sugar fermentation—you can use anything as long as it has sugar or cellulose in it.”
Chemical methods offer a broader platform from which to operate, Mascal says, “because chemically manipulating carbohydrates, as opposed to fermenting sugars, allows you to make alcohols, esters, and furans from a single starting point that can be used to make different types of transportation fuels.”
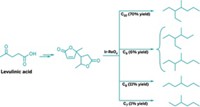
One of the primary pathways to biofuels is aqueous-phase chemistry. Mascal’s group has developed a biphasic acid/solvent reactor to make substituted furans from a cellulosic feedstock in a single step, which eliminates the need to first pretreat or break down the biomass, a step normally required for solution-phase chemistry. The researchers use a hydrochloric acid solution to digest the cellulosic starting material, continuously extracting the reaction mixture with dichloroethane to obtain the substituted furan 5-(chloromethyl)furfural, a biofuel intermediate.
The team has evolved the process to convert biomass crops such as grasses or waste biomass such as corn stover, wood, straw, and recycled paper into either 5-(chloromethyl)furfural or another biofuel intermediate, levulinic acid, depending on reaction conditions, in yields up to 95% (Green Chem., DOI: 10.1039/b918922j). “To our knowledge, this level of conversion of carbohydrate feedstocks into simple organic molecules is unrivaled,” Mascal says.
As an added benefit, the single-reactor processing doesn’t give off any carbon dioxide like most biofuel technologies do, he notes. A key scale-up issue for Mascal is the low efficiency and poor carbon economy of most biofuel processes, which spells poor economics and contradicts the carbon-neutral goal of biofuels.
Microorganisms readily convert glucose into ethanol, but inefficiently because one-third of the available carbon ends up as CO2, Mascal notes. Plus, there’s a variety of five- and six-carbon sugars in the cellulose and hemicellulose polysaccharides that make up biomass, but yeasts typically used in fermentation consume only the six-carbon sugars. The microbes also work more slowly than an industrial chemical process and can’t tolerate the high levels of ethanol they produce, which limits batch processing.
For producers of biofuels other than ethanol, a significant portion of the carbon is also lost as CO2, compromising the yield of hydrocarbons, Mascal says. For these reasons, he thinks his single-reactor route to furans and others like it have an edge.
One drawback, however, is the halogenated solvent, which might have to be replaced in an industrial-scale process.
When derivatized, the furfural or levulinic acid leads to other furans or levulinate esters that can be used as stand-alone fuels—which would require regulatory approval—or more likely as blend stocks to make traditional gasoline, diesel fuel, or jet fuel. Mascal is exploring opportunities with potential commercial partners—there’s no shortage of interested capital investors knocking on his door—and he has been collaborating with Nevada-based Bently Biofuels to test some biodiesel candidates.
Among biofuel companies, Virent Energy Systems, based in Madison, Wis., was one of the first to bet on aqueous-phase chemistry. Although their process so far begins with sugars rather than cellulosic material, it ends in desirable hydrocarbons rather than oxygenated compounds.
Founded in 2002 by Dumesic and his Wisconsin colleague Randy D. Cortright, Virent is commercializing its BioForming technology. The process employs heterogeneous catalysts at moderate temperatures and pressures in a collection of parallel and tandem reactions to first partially deoxygenate soluble sugar feedstocks into sugar alcohols, which then pass through an aqueous-phase reforming process that converts them into a collection of fuel chemicals. For example, Virent produces a gasoline blend containing primarily C5 to C10 alkanes and aromatics—essentially the same blend of compounds as petroleum-derived gasoline. The company also can produce diesel fuel and jet fuel blends.
One of BioForming’s advantages is that the hydrogen needed is generated in situ, which cuts down costs. In addition, the product hydrocarbons are easily separated from the aqueous phase, saving on distillation costs.
Virent is currently demonstrating the process in a 10,000-gal-per-year pilot plant, marketing director Mary W. Blanchard notes. And as part of the National Advanced Biofuels Consortium, the company aims “to add cellulosic deconstruction technology to our production facilities,” Blanchard says. Virent is on track to be running a commercial-scale biogasoline plant by the end of 2015.
Another primary chemical pathway to biofuels is pyrolysis. On this front, George W. Huber and his group at the University of Massachusetts, Amherst, have developed a continuous catalytic pyrolysis method that directly converts raw biomass such as wood chips into gasoline-range compounds. Huber is a former graduate student of Dumesic’s at Wisconsin and part of the team that developed some of Virent’s technology.
Pyrolysis uses moderate heat and low-oxygen conditions to break down cellulosic material into “biocrude,” a mixture of more than 300 liquid hydrocarbons. “Pyrolysis is the cheapest way to take biomass and make a liquid fuel from it,” Huber says. But it’s tricky: The biocrude is acidic and has high water content, two features that make it unstable and hard to handle, Huber explains. Thus, the oil has to be processed quickly to upgrade it into fuel-range derivatives.
Upgrading can be done by standard refinery chemistry—including catalytic cracking and hydrotreating—that converts the complex pyrolysis compounds into simpler hydrocarbons. Both methods have been extensively tested and developed for decades, Huber says, but only now that the price of crude oil is high is pyrolysis becoming economically viable. A few companies using pyrolysis to make biofuels are edging toward commercial-scale processes.
Meanwhile, Huber’s group is combining pyrolysis with catalytic cracking and hydrotreating to make hydrocarbons directly from raw biomass in a single step. The approach, which Huber has named catalytic fast pyrolysis, quickly converts biomass to biocrude at 600 °C in a specialized reactor. During catalytic fast pyrolysis, fine particles of the zeolite catalyst ZSM-5 mixed with the biomass form aromatic compounds, Huber says. Olefins are a coproduct, and the aromatics-to-olefins ratio can be adjusted by changing reaction conditions. When applied to sawdust in a single fluidized-bed reactor, Huber notes, the technique yields a mixture containing five of the six major petrochemicals used as chemical feedstocks: benzene, toluene, xylene, ethylene, and propylene (Energy Environ. Sci., DOI: 10.1039/c0ee00341g).
“Our challenge now is to scale up the technology,” Huber says. “Just about anything can be done in the lab; but the real question is, can it be done on a large scale, and be cost-competitive with petroleum, and provide enough economic incentive that people are going to want to invest in it?”
To find out, Huber started Anellotech, a company that initially plans to make aromatics as commodity chemicals but also aims to market the aromatics as fuel-blending agents or the combination of aromatics and olefins as a gasoline blend stock or possibly a stand-alone fuel.
Chemical routes to biofuels continue to show commercial promise, but synthetic biology has the potential to do so much more, UC Berkeley’s Keasling says.
In addition to leading his UC Berkeley group, Keasling serves as chief executive officer of the Department of Energy’s Joint BioEnergy Institute (JBEI) in Emeryville, Calif. JBEI is using synthetic biology to develop new bioenergy crops, enhance biomass degradation, and engineer microbes and enzymes to transform sugars into biofuels.
“Current synthetic biology routes to making biofuels use well-known, safe, engineered microorganisms,” Keasling says. “But in the future, production schemes may include designer cells that are tailor-made for the desired chemical or fuel and production process.”
A handful of companies are already zeroing in on commercial biofuels produced by microbes. For example, Gevo, based in Englewood, Colo., uses an engineered microbe to produce 2-methylpropanol, known in the industry as isobutanol, which can be used as a gasoline blend stock or dehydrated to isobutylene and then converted into octane, aromatics, and other gasoline ingredients. The technology is based on research by James C. Liao and coworkers at the University of California, Los Angeles.
Gevo has a 1 million-gal-per-year demonstration plant on-line and expects to have its first commercial-scale plant running this year. That plant will be able to produce up to 50 million gal of 2-methylpropanol annually. Last November, Gevo became the first company to receive the Environmental Protection Agency’s approval to blend a butanol with petroleum-derived gasoline.
Research from Keasling’s UC Berkeley lab has been equally successfully translated into commercial ventures. “We took a look at all the basic biosynthetic pathways that lead to hydrocarbons,” Keasling says. “There are linear-chain hydrocarbons that you get from fatty acid biosynthesis and branched hydrocarbons that you can get from the isoprenoid pathway.” These pathways are the basis of biotech start-up firms LS9 and Amyris Biotechnologies, both located near San Francisco.
LS9’s technology is based on manipulating the fatty acid metabolism of the industrial workhorse bacterium Escherichia coli, effectively capturing in a microbe the machinery that plants use to make vegetable oil, Keasling explains. LS9 scientists are creating engineered E. coli “microrefinery catalysts” such that each selectively makes a single hydrocarbon product with a different chain length, extent of saturation, or degree of branching.
LS9 already has a 1,000-L pilot plant to produce fatty acid methyl esters that can serve as biodiesel. The company is working toward opening a commercial-scale demonstration facility in Okeechobee, Fla., to produce fuels and other products using sugar as a feedstock.
In an extension of LS9’s biodiesel approach, JBEI scientists added to E. coli the ability to produce hemicellulases, which are enzymes that break down hemicellulose (Nature, DOI: 10.1038/nature08721). The research team uses the microbe to depolymerize purified hemicellulose into its constituent xylose sugar molecules and then metabolize the sugar to fatty acid ethyl esters, Keasling says. The development is a first step in “consolidated bioprocessing,” using a single microbe to both break down cellulosic material and convert it into a biofuel, he notes.
Amyris’ technology, meanwhile, is based on an engineered yeast that ferments sugar into the 15-carbon isoprenoid β-farnesene, which can be derivatized to a range of products, Keasling says. The company is developing a way to hydrogenate farnesene to farnesane, which can serve as biodiesel. Amyris has a pilot-plant operation in California and a demonstration plant in Brazil, where it plans to integrate operations with sugar and ethanol producers to ensure steady access to a sugar feedstock.
Advertisement
Both the fatty acid and isoprenoid products diffuse out of the engineered cells, and because they have low solubility in water, they readily separate from the fermentation broth, making purification simple, Keasling says. That ability reduces fuel cost relative to ethanol by avoiding energy-intensive distillation. The products’ low solubility also means that they present low toxicity for the microbes, allowing much higher titers in fermentation tanks than is possible with ethanol and yeasts, as well as higher yields from the sugars, he adds.
On the downside, the microbes don’t produce short, highly branched molecules. “Those are needed for gasoline, but they are a challenge to make biosynthetically,” Keasling says.
Another limitation is that engineered microbes typically produce only one type of molecule, whereas fuels are generally mixtures of many different molecules that collectively provide the qualities that are important for combustion. However, there is no reason that the fuel needs to be so complex, Keasling says. For some ideal molecules with the right properties, the single compound could serve as a stand-alone fuel.
“In the future, it should be possible to engineer a single organism to produce a mixture of molecules,” Keasling adds. “Or maybe we could engineer a consortium of microorganisms, each of which produces a different important molecule for the fuel. One could then tune the fuel properties by tuning the number and activity of each microbe in the consortium. This is a pretty far-out idea, but it might be possible one day.”
As biofuel technologies proliferate, start-ups and investors must decide which ones are technically and economically feasible on a commercial scale. Decision making typically has focused on how to convert the biomass, but it should be refocused on which raw material should be used, argues Bruce E. Dale, a chemical engineer at Michigan State University and a lead scientist at DOE’s Great Lakes Bioenergy Research Center, one of JBEI’s two sister centers.
“The raw material choice ultimately dictates the process,” Dale explains. “For woody materials, thermal processes such as pyrolysis and gasification are the better technologies, because the high lignin content interferes with biological conversion. For grasses and crop residues, biological routes have the inside track because the thermal approaches choke a bit on the high ash generated by grasses.” But when starting with sugars, chemical and biological approaches are on more equal footing.
In addition, the thermal methods need low-moisture biomass, but most biomass isn’t very dry, Dale says. It would cost more energy if the material has to be dried before processing. For biological methods, moisture content is less of a concern, he adds.
Another factor in decision making is the logistics of biomass availability, transport, and storage. To be commercially viable, a gasification plant would require up to about 15,000 tons of biomass per day, Dale says, whereas a fermentation facility would need about 5,000 tons per day and a pyrolysis facility would need about 2,000 tons per day. To win, companies must work out a long-term, reliable feedstock supply, as well as a partner who will take their fuel, Dale says. Those that don’t will take longer to reach the pump or may not go anywhere.
One company that has established upstream and downstream connections is Mascoma, for which Dale serves as a scientific advisory board member. It is using the enzymatic talents of a bacterium that produces multiple cellulose-degrading enzymes and sugar-fermenting enzymes to produce ethanol directly from cellulosic biomass in one step. The company has worked out its long-term supply of wood from Michigan’s upper peninsula, where it is building a 40 million-gal-per-year ethanol plant. Petrochemical firm Valero Energy, one of Mascoma’s investors, has signed on to purchase all the cellulosic ethanol the plant produces.
So which technologies are primed to take the biofuels market by storm?
Vinod Khosla, cofounder of computer company Sun Microsystems (now part of Oracle), has become the most widely recognized investor in sustainable energy technologies through his company Khosla Ventures. Comments from Khosla make investors and scientists perk up and take notice.
At a GoingGreen conference held in San Francisco last October, Khosla noted that “radical innovation, not incremental improvement, is needed to make clean, efficient energy technologies that can compete, unsubsidized, in big markets.” That ideology applies to the biofuels industry, he said.
Khosla thinks that biofuels are one of the most interesting areas in the energy marketplace. That’s because, unlike electric cars, biofuels will be affordable most everywhere. Still, perhaps only half a dozen or so biofuel approaches will win, he said.
For example, biodiesel from palm oil might be a good idea, Khosla noted. But it may well disappoint investors because it’s just an incremental improvement and unlikely to come out on top. “Other people are trying to make magic work where it hasn’t worked before, such as using algae to make fuel,” he added. “I’ve looked at two dozen business plans, and I haven’t found one where the economics will work.”
Khosla instead has his bets on Gevo, LS9, and Amyris. That’s because all three companies have innovative biofuel technologies that they can also use to make high-margin chemical products, thereby reducing risk.
UMass’s Huber thinks pyrolysis will be a big winner, with different technologies for making gasoline, diesel fuel, and jet fuel. Acid hydrolysis or enzymatic methods will be too expensive on a large scale, Huber believes. “The enzymes cost too much, and fermentation is very slow. If you use acids, then you have to pay to dispose of the acid or you have to try to recycle the acid.”
To get around their tortoiselike reputation, biological approaches would have to run on a larger scale. By some estimates, the U.S. would need 4,000 1 million-gal fermenters to provide enough biofuels to meet demand in a petroleum-free world. But at the same time, fermentation tanks are cheap, whereas chemical reactors are not.
“We are in a commodity market,” Huber says. “At some point, it is going to be a function of economies of scale. There are lots of biofuel technologies being developed, and all of them are achievable. There needs to be some policy to help drive their development. But it has to be done in a way that is technology agnostic, that doesn’t favor one technology over another. There will be winners and losers, but let’s let the market decide, not the politicians, and use our resources as efficiently as possible.”
“When the smoke clears, I think there will be a few technologies left standing,” Mascal agrees. “They will be the ones that can be done cheaply and in which feedstock supplies and their transportation aren’t an issue, capital and operating expenses aren’t prohibitive, and you get a product with a ready or emerging market. The more esoteric methods will be history in the literature.
“But scientists and engineers, or the federal government for that matter, don’t need to pick winners or losers,” Mascal continues. “The market does that itself, and science does that itself. We recognize when one method is much better than another; that’s a type of natural selection.”
“We spent decades getting petroleum-based fuels up to the volumes that we are producing now,” Keasling notes. “With new biofuel technologies, we can’t expect that to happen overnight. But in the next couple of years, we are going to see some of these advanced fuels on the market. From there it will continue to grow. But we’ve got to give it time.”










Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter