Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
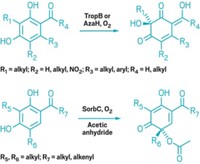
Synthesis
Big Enzyme Takes On Tiny Target
Catalysis: Modifying a huge active site enables conversion of methane to methanol
by Sarah Everts
February 28, 2011
| A version of this story appeared in
Volume 89, Issue 9

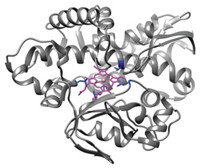
Chemists in Germany have discovered a way to persuade a cytochrome P450 (CYP), an enzyme that normally oxidizes large molecules such as fatty acids, steroids, and terpenoids, to do its work on the tiniest of alkanes, methane. The team, led by Manfred T. Reetz at the Max Planck Institute for Coal Research, in Mülheim, Germany, induced CYP to work on the exceptionally small target by introducing a chemical “guest” into the active site to fill the unused space (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201006587).
Methane is at least an order of magnitude smaller than CYP’s usual substrates, and it doesn’t have a high probability of orienting properly for oxidation to occur at CYP’s catalytic heme active site. To fill the unwanted space, Reetz’s team used a perfluorocarboxylic acid, which also helps push the reaction forward by partly converting the catalytic iron species in the active site from low spin to the catalytically active high spin.
The work provides “a novel, readily applicable handle by which to tune the reactivity and selectivity of both natural and engineered enzymes,” says M. Christina White, a chemist at the University of Illinois, Urbana-Champaign.
The work also offers new inspiration for turning methane into methanol, an attractive alternative to oil or natural gas feedstocks for making fuels and organic chemicals. Turning methane into methanol can be tricky because methane has the strongest C–H bond of any alkane. Reetz’s team “has shown for the first time that heme iron enzymes can generate an oxidant capable of oxidizing methane. These findings are certain to influence future efforts to develop practical enzymatic systems for industrial processes” to turn methane into methanol, White says.
Reetz aims to adjust the active sites of other enzymes so they can accommodate other reactants. He plans to use the “guest” strategy in combination with directed evolution to enable enzymes to transform nonnative substrates.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter