Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
A Simple Test For Botulism Toxin
Homeland Security: A new fluorescence-based assay could find use as a field test
by Rajendrani Mukhopadhyay
February 28, 2011

Botulism is a rare but devastating paralytic illness caused by a toxin made by the bacterium Clostridium botulinum. The Centers for Disease Control and Prevention has identified botulinum toxin as a potential bioterror agent when laced into food supplies. Current detection methods for the bacterial toxins are time consuming and require laboratory settings. Researchers have now come up with a quick and easy assay that has the potential to be a field test (ACS Nano, DOI: 10.1021/nn102997b).
Conventional approaches to detecting botulinum toxin involve either testing samples on mice or performing complicated immunoassays. Analytical chemist Kim Sapsford at the Food and Drug Administration and her husband, molecular biologist Igor Medintz at the Naval Research Laboratory, led a team to develop a simpler and quicker assay for one of the seven C. botulinum toxins, botulism neurotoxin type A. Type A is the most potent and well-studied of the seven toxins and, according to CDC, is the most common cause of foodborne botulism in the U.S. Inside humans, the toxin attacks a protein that plays an important role in nerve-cell communication.
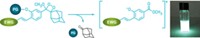
For their assay, the investigators created a synthetic version of the protein sequence that type A toxin attacks and tagged it with a fluorescent dye called Cy3. They incubated the peptides with solutions of the toxin in varying amounts. Next, they added quantum dots, which are semiconductor nanocrystals that absorb and emit light. The peptides spontaneously grabbed the quantum dots.
The investigators then did a fluorescence resonance energy transfer experiment, known as FRET, to calculate the toxin's concentration. They used light to excite the quantum dots into a higher energy state. If type A toxin was absent, the dye on the peptides accepted energy from the quantum dots and fluoresced. But if the toxin was present, it would chew up the peptide, separating the dye from the dots. As a result, the peptide-quantum dot complex did not fluoresce. The researchers inferred that higher fluorescence intensity meant lower concentration of toxin.
The investigators demonstrated that the method could detect as little as 350 pM of the toxin, which is comparable to the immunoassays' sensitivity. Sapsford and Medintz emphasize that their assay represents an improvement because it measures the active form of type A toxin; immunoassays measure the toxin's presence but not whether it's active. Only the active form poses a threat to security. But the new assay is far less sensitive than the mouse test, which detects as little as 0.1 pM but also kills the animals.
Sapsford and Medintz also say that the assay components can be easily packaged into a microscale platform for field tests. That potential for portability is one of the best attributes of the work, says chemical biologist Tobin Dickerson at the Scripps Research Institute. He also applauds its three-hour timeframe, which beats conventional methods'. But because the current work only looked at simple solutions of the toxin, Dickerson cautions that the assay's ability to detect the toxin may decrease in more complex samples, like milk.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter