Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Proteins Reveal Their Secrets Under Pressure
NMR Spectroscopy: New device allows chemists to apply pressure to proteins during NMR experiments
by Erika Gebel
August 1, 2011

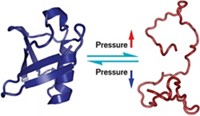
Putting pressure on proteins can make them adopt rare conformations that reveal secrets about how they bind ligands or catalyze reactions. Now researchers have developed a device that can apply such pressure to proteins during nuclear magnetic resonance spectroscopy experiments, providing chemists with atomic-level details about important conformations (J. Am. Chem. Soc., DOI: 10.1021/ja2050698).
Proteins aren’t static molecules; they fluctuate between structural states in milliseconds. Unfortunately, these transient protein conformations are rare under normal conditions, so it’s difficult to collect structural information on them.
Researchers have long known that applying pressure to proteins can stabilize these rare protein conformations and make them last longer. They study these fleeting states using a variety of methods, such as ultraviolet/visible spectroscopy and fluorescence spectroscopy. Techniques like NMR could provide scientists with atomic-level data on these conformations, but applying high pressures to samples quickly and safely inside an NMR spectrometer is daunting, says Hans Robert Kalbitzer, of the University of Regensburg, in Germany.
Kalbitzer and his colleagues found a solution: They developed a small device they call a pressure jump unit, which consists of a series of valves, two pressure sensors, and a pump. The unit sits outside the spectrometer and, through tubing, controls the pressure of a sample inside the NMR spectrometer. With careful optimization, in less than 30 ms, the unit can apply 80 MPa of pressure, which is about 790 times greater than atmospheric pressure.
With the device, the researchers attempted an NMR experiment they call pressure perturbation transient state spectroscopy. The experiment begins with a pressure spike that unfolds a protein in solution in an NMR sample tube; then the protein refolds under normal pressure. The resulting NMR scans provide kinetic data about the folding process.
They tested HPr, a protein that Kalbitzer’s team had studied before. At normal pressures, HPr is in its active, folded state, while high pressure causes it to switch to its inactive, unfolded state. In the pressure experiments, the NMR spectra of these two states closely matched spectra from previous, non-pressure experiments on HPr. But the technique also allowed the team to determine the rates of folding and unfolding, which was previously impossible to measure by NMR. Adding structural information to their kinetic results, the researchers could combine pressure jumps with standard NMR experiments to provide data on the connectivity of atoms within HPr.
A. Joshua Wand, of the University of Pennsylvania, is excited about the new device, pointing out that NMR has not worked well to study protein folding under pressure. Now, he says, chemists can watch proteins reversibly unfold and refold at a level of detail that only NMR can provide.
While the pressure jump unit isn’t currently commercially available, Kalbitzer says he has spoken with several companies about the possibility.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter