Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Cellular Sodium-Calcium Exchange Illuminated
Probe of membrane protein structure deepens understanding of critical cellular maintenance functions
by Jyllian Kemsley
February 13, 2012
| A version of this story appeared in
Volume 90, Issue 7
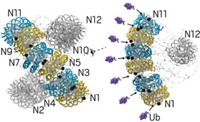
A clearer picture of how cells traffic calcium comes from a new structure of a membrane ion-exchange protein (Science, DOI: 10.1126/science.1215759). Cellular calcium transport is essential for processes including fertilization, muscle contraction, and cell death. A group led by Youxing Jiang, a Howard Hughes Medical Institute investigator at the University of Texas Southwestern Medical Center, crystallized and solved the structure of an archaeal Na+/Ca2+ exchanger (NCX). The protein is homologous to eukaryotic transport proteins that exchange three Na+ ions for one Ca2+ ion across cell membranes. The group found that NCX is made up of a bundle of 10 helices that span a membrane and enclose a central cation-binding pocket. The researchers propose a “helix sliding” conformational mechanism for ion exchange that alternately opens ion channels to the interior and exterior of a cell, with Na+ and Ca2+ ions competing for ligands in the binding pocket. The structure generally confirms what scientists had learned about eukaryotic exchangers through other methods. The archaeal protein, however, does not include a regulatory domain found in eukaryotic exchangers, so interactions between the regulatory and transport domains remain to be clarified.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter