Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Delivery
Protein Drugs That Are Easy to Take
Biopharmaceuticals: Crowded protein nanoclusters produce highly concentrated, injectable suspensions of the drugs
by Katherine Bourzac
January 27, 2012

Protein-based drugs are a fast growing class on the pharmaceutical market, with thousands of candidates in development. But because administering proteins to patients at sufficient doses sometimes is difficult without an intravenous drip, many potential drugs haven’t reached the clinic. To overcome this problem, researchers have developed a way to make highly concentrated, injectable suspensions of proteins (ACS Nano, DOI: 10.1021/nn204166z).
“Drug-delivery technology has not kept up with protein discovery,” says Keith P. Johnston, a professor of chemical engineering at the University of Texas, Austin. Proteins can’t be taken in pill form. And because highly concentrated protein solutions become viscous, injections under the skin often aren’t feasible. When a patient needs a high dose of a protein, they usually need an intravenous drip. For example, some rheumatoid arthritis patients must go to a clinic for regular intravenous treatment, an expensive, stressful process that can last several hours.
Johnston’s group wanted to find a way to pack a high dose of protein drugs in a syringe, to allow simple injections at the doctor’s office or at home, as with insulin.
To start, Thomas Truskett, one of Johnston’s collaborators, modeled proteins in solution. He found that because the charged molecules attract one another, they start to unfold and clump up at concentrations around 100 mg/mL. But Truskett noted that in cells, where proteins abound at concentrations four times as high, they don’t form clumps.
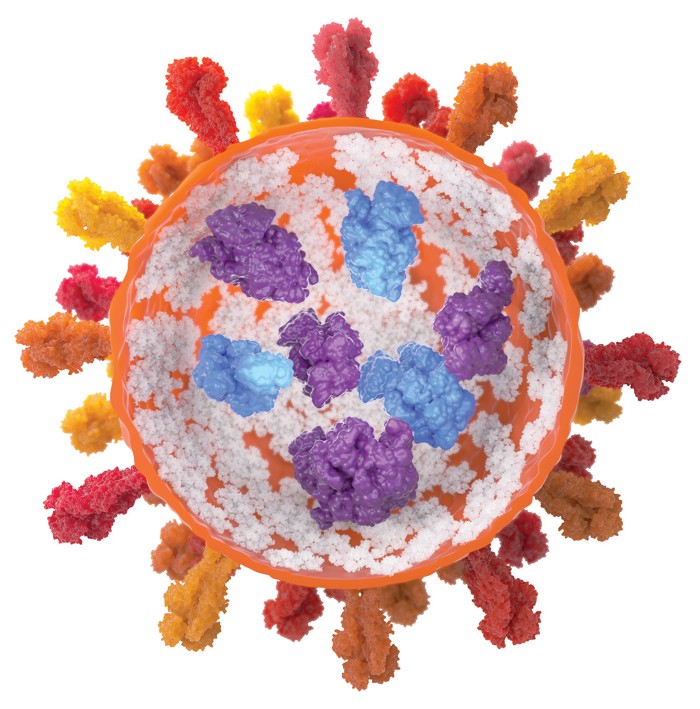
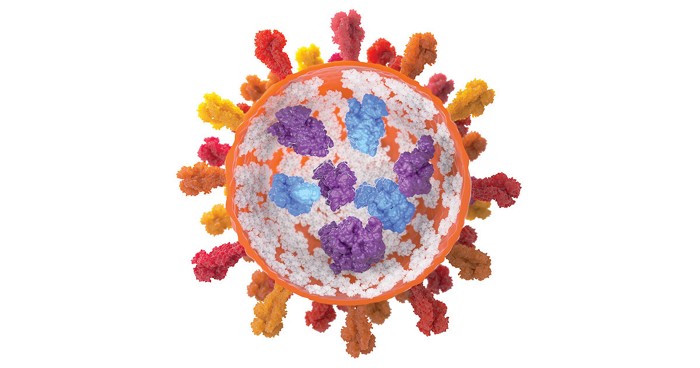
Johnston and his team wondered if a way to keep the proteins from aggregating at high concentration was to mimic the highly crowded environment inside the cell. They thought they could do that by encouraging the proteins to aggregate into nanosized clusters.
To produce these nanoclusters, the researchers first adjusted the pH of the protein’s solution so that the molecules have almost no net surface charge. Then they added trehalose, a nontoxic sugar, in sufficient quantities to push the proteins together. With no surface charge, the proteins don’t repel one another and they form nanoclusters a few hundred nanometers across. By increasing or decreasing the amount of sugar, the researchers could shrink or grow the size of the nanoclusters.
The scientists characterized nanocluster suspensions of three different proteins at concentrations as high as 700 mg/mL. For a batch made with an antibody that binds the pertussis toxin, they found that the clustered antibodies remained active. After diluting the suspensions in a tube to free the antibodies, the researchers found that the proteins bound the toxin with the same affinity as proteins from a conventional solution. When they injected the suspension under the skin of mice, the nanoclusters took longer to diffuse into the bloodstream than did conventional protein solutions, but once in the bloodstream, both protein formulations had similar activities and lifetimes.
Theodore W. Randolph, of the University of Colorado, Boulder, says that to demonstrate that the nanocluster method is practical, the researchers will have to show that the nanoclusters have at least a two-year shelf life, and that the method works for other proteins. The Texas group is now working with two pharmaceutical companies on further tests.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter