Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Gold Cleans Up Mercury
Water Treatment: Researchers reverse gold-mining process to rid water of mercury
by Katharine Sanderson
February 23, 2012

Taking inspiration from a centuries-old-process for extracting gold from ore, researchers have used gold nanoparticles to strip mercury from contaminated water (ACS Nano, DOI: 10.1021/nn204313a).
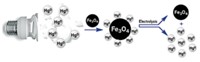
Chemists have looked to nanoparticles to remove a range of metals from dirty water, in part because of the particles’ high surface areas. Victor Puntes at the Catalan Institute of Nanotechnology in Barcelona, Spain, thought gold particles could pull out mercury. He recalled that miners used to make mercury-gold amalgams to get gold out of ores. So he and his team wanted to reverse the process to take mercury out of contaminated water.
They knew that to make a mercury-gold amalgam, they would have to reduce the mercury ions in water. But a strong reducing agent, such as sodium tetrafluoroborate, would reduce any metal in solution, clogging the surfaces of the gold nanoparticles with other metals aside from mercury.
Fortunately, the researchers stumbled upon a simple solution. When they produced 9-nm-diameter gold particles, the scientists coated them with sodium citrate to keep the particles dispersed in solution. Citrate also is a mild reducing agent, capable of turning mercury ions into elemental mercury.
As a result, the gold nanoparticle surface acts as a catalyst, Puntes says, to reduce mercury ions when the researchers mix nanoparticles with mercury-spiked water. Mercury quickly adsorbs onto the gold surfaces, and over two days the metal moves inside the nanoparticles, forming an amalgam. The researchers can then centrifuge the water to remove these mercury-heavy nanoparticles.
Puntes and his team first tested their method on pure water spiked with mercury chloride. The nanoparticles could remove all mercury ions from the solutions up to a mercury ion concentration of 0.16 ppm, resulting in water that meets the World Health Organization’s safety guidelines of 1 ppb. At a mercury concentration of 4.8 ppm, the particles pulled out about 60% of the mercury. To test a more realistic solution, the scientists then added mercury ions to water samples from the nearby Ebro River. Their method could remove about 40% of the mercury ions from a 6.5 ppm solution.
Although he says that the method is novel, Mamadou Diallo of the California Institute of Technology, thinks that the water industry is unlikely to use it to remove mercury because of gold’s high costs. “I think this will remain a lab curiosity,” Diallo says. “There are other less expensive methods for extracting mercury from water,” he adds, including using thiol molecules to scavenge for mercury ions.
But Puntes hopes that the gold nanoparticles could remove traces of mercury in water that has already gone through several treatment steps. “Cleaning very dirty water is very easy,” he says, “but cleaning cleaner water is more difficult.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter