Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Large Semiconductor Membranes Made In A Dish
Nanofabrication: A simple, cheap, and scalable way to produce nanomembranes for flexible devices
by Prachi Patel
February 16, 2012

Researchers have demonstrated a simple, cheap technique to grow large, ultrathin semiconductor sheets on the surface of water (ACS Nano, DOI: 10.1021/nn2050906). This method holds promise for producing flexible electronics, light-emitting diodes, and medical sensors, the researchers say.

Scientists prefer thin films, or nanomembranes, to nanocrystals and nanowires, because they can easily integrate them into electronic devices. However, making a semiconductor nanomembrane is expensive and time-consuming. The process involves growing it on a substrate—for example, growing a silicon film on silicon dioxide—and then etching away the substrate to release the membrane. Scientists are also limited in the types of materials they can make, because the membrane’s crystal structure must match that of the substrate. So far scientists have made nanomembranes from only a few semiconductors, such as silicon and gallium arsenide.
Xudong Wang, a materials science and engineering professor at the University of Wisconsin, Madison, wanted to find a simpler way to make large nanomembranes. His material of choice is zinc oxide, which is a promising material for flexible transistors and ultraviolet light-emitting diodes. Wang also wants to exploit zinc oxide’s piezoelectric property of converting mechanical stress into electric current. His goal is to make tiny generators that harness muscle movements or blood flow to power medical sensors.
For their new production method, Wang and his colleagues ditched the crystalline substrates and drew inspiration from a surfactant-based approach for making nanocrystals. In that method, surfactants form a layer on the surface of water and act as a template for growing nanocrystals. Scientists have made micrometer-wide particles with this approach.
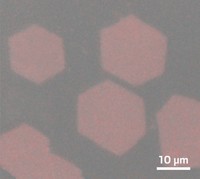
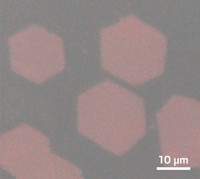
By finding the right combination of chemicals, Wang and his group developed a process to make millimeters-wide films. They mix two zinc oxide precursors in water in a dish and then add a high concentration of the surfactant sodium dodecylsulfate. In a few hours, the water surface is covered with a membrane of zinc hydroxy dodecylsulfate a few hundreds of nanometers thick. The membrane consists of overlapping hexagonal plates of the material.
“It’s amazing that they can grow such large two-dimensional membranes,” says Jiaxing Huang, a materials science and engineering professor at Northwestern University. He notes that the key to growing the large sheets was the researcher’s “perfect combination of surfactant and precursors.”
The researchers could scoop up the zinc hydroxy dodecylsulfate membrane using a silicon or carbon substrate and heat it to give a zinc oxide film. The team also constructed transistors by transferring the zinc hydroxy dodecylsulfate film to a plastic substrate and patterning electrodes on it. Wang says the zinc oxide film still has some sulfate left over from the surfactant, and he’d like to make it purer and thinner.
Weidong Zhou, an electrical engineering professor at the University of Texas, Arlington, says the new technique is more cost effective than the existing methods. It could scale up to produce very large films, he says, “opening a door to a wide variety of functional device applications.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter