Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Algal Toxin Breaks Record
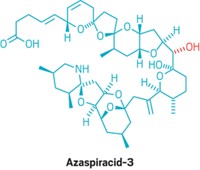
Natural Products: The molecule has the largest number of contiguous polycyclic ether rings—17—of any marine algal toxin
by Melissae Fellet
March 5, 2012

For three months in 1998, a red tide blanketed parts of the southern coast of New Zealand’s North Island. A new algae species, Karenia brevisulcata, in the bloom spewed toxins that killed most fish in Wellington Harbor and caused respiratory problems in more than 500 people nearby. Now chemists have determined the structure of one of the toxins and noted that it has the longest contiguous polycyclic ether structure ever observed in an algal toxin (J. Am. Chem. Soc., DOI: 10.1021/ja212116q).
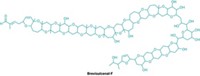
Scientists had previously isolated groups of toxins from this species, but the large size of many of the molecules made complete structural determination difficult. Masayuki Satake, of the University of Tokyo, and his colleagues have isolated and characterized one of the most prevalent toxins, brevisulcenal-F.
The team isolated the compound by passing extracts of the algae through several rounds of chromatography, picking only the fractions that killed mouse leukemia cells. They then determined the molecule’s structure using high-field two-dimensional nuclear magnetic resonance spectroscopy to resolve the many signals that overlap because of the molecule’s size. To confirm their NMR data, the chemists also used tandem mass spectroscopy.
Brevisulcenal-F contains 17 contiguous ether rings, making it the longest polycyclic ether system of any marine algal toxin. The previous record holder was gymnocin-B, with 15 fused rings. Another string of five connected ether rings attaches to the brevisulcenal-F core by a flexible carbon string. An aldehyde dangles from the other end of the core.
The pendant aldehyde and ether rings are common in marine algal toxins. Comparing the structure of brevisulcenal-F to that of other known toxins, such as gymnocins and brevetoxins, will help scientists determine how these common features influence each toxin’s potency, Satake says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter