Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Picture-Perfect Particles Enhance MRI Signal
Cell Imaging: Giant lipid vesicles fill cells with contrast agents
by Katharine Sanderson
April 13, 2012

By stuffing magnetic nanoparticles into giant lipid vesicles, scientists have tracked single cells with magnetic resonance imaging (Anal. Chem., DOI: 10.1021/ac2031354). The technique, they say, is easy and causes little harm to the cells.
With MRI, researchers would like to pinpoint specific cells in a living organism and watch them in real time. This information could help them understand the progression of disease cells or the fate of treatments such as implanted stem cells.
To highlight these cells, scientists need to use chemicals called contrast agents. These compounds change how nearby atoms respond when put in the external magnetic field produced by an MRI machine. The result is these atoms produce distinct signals that researchers can spot in an MRI scan.
Unfortunately, current contrast agents have a couple of problems: It is difficult to deliver enough contrast agent into a cell to produce a noticeable signal, and many of these compounds are toxic to the cells.
Ichio Aoki of the National Institute of Radiological Science, in Anagawa, Japan, and his colleagues tackled both of these problems. To deliver enough contrast agent into a cell to produce strong signals, they packed nanoparticles of iron oxide, a known contrast agent, inside giant lipid vesicles. The researchers thought that cells would absorb these vesicles, thus pulling in the right amount of contrast agent. Previous studies have found that iron oxide nanoparticles are less toxic than other contrast agents. The researchers thought the vesicles would also provide a temporary barrier between the particles and the cells.
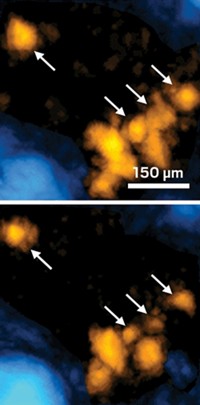
Aoki and his team made the vesicles by mixing a suspension of iron oxide nanoparticles in water with lipids. The resulting emulsion contained nanoparticles wrapped in lipid membranes. They then centrifuged this mixture to force the membranes to fuse, creating giant bubbles 4.7 µm wide and filled with the contrast agent.
To test the vesicles, the researchers injected them into medaka fish (Oryzias latipes) embryos and then placed the embryos inside an MRI scanner. They could easily spot cells containing a single giant vesicle in the resulting images. “A giant vesicle can carry enough of the nanoparticle contrast agent to induce a big black spot on the image,” says Aoki.
To test the vesicles’ toxicity, the researchers compared the vesicle-injected embryos to ones they injected with just saline. After 48 hours, there was no difference in the number of cellular abnormalities between the two groups of embryos.
Jeff Bulte, an expert in imaging technologies at Johns Hopkins University, says that the high load of nanoparticles in the vesicles certainly improves the sensitivity of MRI signals in single cells. He would like to see how long the vesicles stay intact inside mammalian cells and if they will allow scientists to track cells for a couple of days.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter