Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Carbon Nanotubes Highlight Tumors
Medical Imaging: The materials could aid cancer detection using a safe, cheap imaging method
by Prachi Patel
June 1, 2012

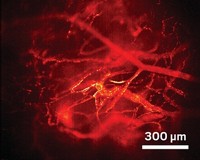
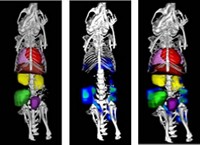
Doctors rely on magnetic resonance imaging and X-ray computed tomography to spot tumors and monitor cancer treatment. But some researchers think a cheaper and safer alternative could come from photoacoustic imaging, a noninvasive technique that produces images based on sound. Scientists have now developed photoacoustic contrast agents based on carbon nanotubes that home in on tumors in mice, highlighting the tissue in scans (ACS Nano, DOI: 10.1021/nn204352r).
To create an image of tissue using photoacoustics, researchers first shine visible or near-infrared light on a stretch of skin. The light heats up tissue and blood beneath the skin. As the tissue warms, it expands and contracts, generating sound waves. Based on features of these waves, computer software then recreates an image of the tissue. These images’ resolution matches those of MRI or CT scans, but unlike those standard methods, photoacoustic imaging doesn’t require expensive equipment or harmful X-rays.
The protein hemoglobin is very good at absorbing near-infrared light, so blood produces stronger photoacoustic signals than most other tissues. As a result, most imaging studies have focused on blood vessels, says Sanjiv Gambhir of Stanford University, who led the new study. To help image other structures in the body such as tumors, scientists have recently started to develop photoacoustic contrast agents. These materials are even better at absorbing light than hemoglobin. What’s more, researchers can decorate contrast agents with molecules that specifically bind to cancer cells or tumor blood vessels. These tags allow agents to accumulate in the tumor, highlighting it with a strong photoacoustic signal.
Gold nanoparticles have been researchers’ contrast agents of choice. Gambhir and his team thought that carbon nanotubes would be a better choice because they are easier to modify. They envisioned producing multiple nanotube-based agents, each labeled with a different molecule that targets one of many cancer-specific proteins. By superimposing images created by signals from each contrast agent, doctors could, for example, look for multiple tumor-specific proteins at once, and thus improve the accuracy of their diagnoses.
To make their contrast agents, the scientists attached one of two dyes to the nanotubes to enhance their ability to absorb light. Then, using a maleimide-containing linker molecule, they tagged the nanotubes with a peptide that binds to a protein on tumor blood vessels.
As a proof-of-principle test, the researchers injected a few micrograms of each type of nanotube contrast agent into mice with brain tumors that the scientists had inserted under the skin of their flanks. By shining light of a wavelength absorbed by either dye, they could produce clear images of the tumors. Without any injected nanotubes, the tumors were only faintly visible in the photoacoustic images. Peptide-covered nanotubes produced two- to threefold higher signals than those without the tumor-specific peptide.
“This is great work,” says Lihong Wang, a photoacoustic imaging researcher at Washington University in St. Louis. Using two light wavelengths to image a tumor is novel for photoacoustic imaging, he says. He thinks that, because modifying carbon nanotube contrast agents is easy, researchers could coat the materials with drugs. These materials could detect cancer and treat it at the same time.
Gambhir says his next step is to test the toxicity of these nanotube contrast agents in animal experiments.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter