Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Benzenes Reduced By Super Electron Donors
ACS Meeting News: Neutral organic molecules transfer electrons to arenes with unprecedented ease, creating reactive intermediates
by Stephen K. Ritter
August 20, 2012
Benzene and other arenes are normally among the most challenging organic species to reduce. But not so for John A. Murphy of the University of Strathclyde, in Scotland. Murphy and his colleagues have devised powerful new versions of neutral organic molecules that readily give away their electrons to reduce arenes and form reactive intermediates, he reported yesterday at the American Chemical Society national meeting in Philadelphia. These reagents, called super electron donors, are on track to become important synthetic tools for organic chemists.
Few chemical reagents have the wherewithal to add an electron to a ground-state aromatic ring to form a radical anion. This type of electron transfer has traditionally required the most brutish of metal-based reducing agents, such as sodium metal dissolved in liquid ammonia.
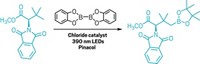
Murphy’s group originally discovered that a simple organic molecule, a bisimidazolylidene, was up to the task of reducing iodoarenes and arenesulfones. In Philly, Murphy’s team reported that shining ultraviolet light on the bisimidazolylidene or a bispyridine analog (both shown) promotes an electron to a higher energy level in the donor, generating the most powerful super electron donors to date (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201200084). The light-activated bisimidazolylidene reduces the more difficult chlorobenzenes to nonchlorinated benzenes, and both light-activated super electron donors can ring-open diphenylcyclopropanes (shown).

“This is the first time any neutral organic reducing reagent has tackled these highly formidable reduction reactions,” Murphy told C&EN. “The sky is now the limit for what simple organic donors can reduce.”
Murphy has set his sights on applying the new reagents to challenging industrial processes such as reducing carbon dioxide to methane and nitrogen to ammonia.
Armido Studer, a expert in radical chemistry based at the University of Münster, in Germany, is intrigued by the potential of the light-activated approach. “Arene reductions by electron transfer are generally conducted with alkali metals dissolved in liquid ammonia,” Studer told C&EN. “That type of reaction, known as the Birch reduction, is well-known in industry and in academia. It’s difficult to compete with the Birch reduction. But just shining light on a molecule to increase its reduction power is certainly easier than playing around with substituent effects. Murphy and his team have shown that light can bring us closer to the established reagents.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter