Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Boxy Compound Wraps Up Pollutants In Neat Packages
Supramolecular Chemistry: A cyclic compound sequesters polycyclic aromatic hydrocarbons of varying shapes and sizes
by Erika Gebel
September 12, 2012

Polycyclic aromatic hydrocarbons are an important class of fused-ring natural compounds that are under fire for being toxic. Evidence suggests PAHs, which occur naturally in oil (including edible oils), coal, and tar, may cause cancer or mutations in humans. Now, researchers have synthesized a chemical that sequesters a wide range of PAHs and may help remove the toxins from the environment (J. Am. Chem. Soc., DOI: 10.1021/ja307360n).
Over the years, scientists have studied several chemicals that bind to PAHs, says J. Fraser Stoddart of Northwestern University, but each has drawbacks. Some are expensive while others can bind to only a limited subset of PAHs. Stoddart’s laboratory had been studying a positively charged molecule made up of six cyclically linked phenyl and pyridine rings that could host a wide range of molecules, including the simplest PAH, the two-ring molecule naphthalene (Angew. Chem. Int. Ed., DOI: 10.1002/anie.198815471). Graduate student Jonathan Barnes and postdoctoral fellow Michal Juríček wondered whether their host molecule could accommodate larger PAHs if they added two more rings to the loop.
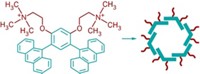
The researchers and their colleagues synthesized an eight-ring version they call ExBox using standard chemistry and inexpensive starting materials, Stoddart says. It dissolves in either water or oil, depending on the choice of anion.
The researchers mixed solutions of ExBox and 11 PAHs, ranging in size from azulene, a simple two-ring structure, up to coronene, which contains seven fused rings. They observed that when ExBox binds a PAH, the solution changes from colorless to yellow, orange, red or brown. The team believes the compound could enable detection of PAHs via a simple visual color change. The researchers crystallized the colorful complexes and solved the structures, verifying that ExBox has a box-like geometry and can encapsulate all 11 PAHs by subtly flexing its architecture to optimize electron sharing.
“It can really adjust to take anything within reason,” Stoddart says. “It’s one size fits all.”
To test whether ExBox maintains its affinity for PAHs in complex mixtures, the researchers added an ExBox solution to a sample of crude oil. After they shook the vial, the aqueous layer quickly turned yellow, suggesting to them that ExBox had captured PAHs. Next, the researchers removed the yellow solution with a pipette and added to it dichloromethane, which binds to PAHs more tightly than ExBox does, releasing ExBox for future use. After evaporating the dichloromethane, they found that ExBox had pulled 1.2 mg of PAH out of 150 μl of crude oil, concluding that they had removed the majority of PAHs.
“It’s some very impressive work,” says chemistry professor Isiah Warner of Louisiana State University, Baton Rouge, whose group focuses on environmental analyses. He says that ExBox is unique in encapsulating a range of polycyclic aromatic compounds. Another strength, he says, is that it works in water, unlike other PAH sequestering reagents such as cyclodextrin. He expects the study will “generate a lot of interest” for sequestering pollutants.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter