Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Light-Powered Molecular Glue Pulls Open Lipid Membrane
Molecular Machines: Compound combines a light-sensitive azobenzene hinge with sticky guanidinium-tipped feet to increase membrane’s porosity
by Melissae Fellet
September 17, 2012

A light-powered molecule disrupts a lipid membrane enough to let ions pass through (J. Am. Chem. Soc., DOI: 10.1021/ja3074424). The “molecular glue,” so called because it sticks to charged surfaces, could open cell membranes, allowing scientists to study how ionic signals travel through cells, the researchers say.
The molecule relies on light-driven isomerization of azobenzene, which other researchers have used to power molecular machines. A flash of ultraviolet light switches the molecule’s central nitrogen-nitrogen double bond from its extended trans conformation to a contracted cis arrangement. This switch creates mechanical motion that researchers have harnessed to flap a molecular paddle or lift a molecular piston.
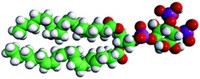
Kou Okuro, Takuzo Aida, and their colleagues at the University of Tokyo thought they could translate azobenzene motion into frictional forces between two surfaces. Those forces help muscles contract when one muscle fiber slides along another. To do this, the researchers built a tweezer-like “molecular glue” using azobenzene as a hinge. Six sticky feet, tipped with positively charged guanidinium ions, adhere the molecule through hydrogen bonds and electrostatic attraction to negatively charged surfaces.
The researchers attached the glue to the outside of a liposome, which is a bubble encased by a lipid membrane; The glue molecule’s feet clung to the negatively charged phosphate groups of the lipid membrane. Then the researchers shone UV light on the complex, which they knew would make the azobenzene hinge flip its conformation, moving the sticky feet of the glue closer together or farther apart.
To see what effect this change had on the membrane, the researchers filled the liposomes with a pH-sensitive fluorescent dye that would light up if hydroxide ions seeped through the lipid membrane due to an opening caused by the glue’s isomerization. The fluorescence intensity increased as the glue feet moved. It leveled off once the glue stopped isomerizing and the ions stopped flowing.
The researchers think that the motion of the glue feet during isomerization tugs on the lipids in the membrane, introducing enough disorder for ions to pass through. They ruled out the possibility that one isomer naturally disrupts the membrane more than the other conformation does by showing that membrane fluidity is similar for each conformation.
Scientists can control membrane ion permeability, and thus some biochemical pathways, by engineering a cell to express light-activated ion channel proteins. This molecule might represent a general way to control ion permeability without genetic engineering, Okuro says. Engineering altered ion channels is possible in cultured cells, he adds, but it’s harder to do in animals.
Christopher Barrett, at McGill University, is pleased to see a light-powered molecule that sticks reversibly to a membrane like a sticky note. Adhering the glue to the membrane using hydrogen bonds instead of covalent bonds improves the molecule’s biocompatibility because the softer interactions resemble reversible interactions in biology, he adds. But the glue requires a lengthy synthesis that may hinder its widespread use, Barrett says.
Nobuyuki Tamaoki, at Hokkaido University, in Japan, is intrigued by the idea of harnessing the isomerization motion. But he contends that the researchers did not effectively rule out that the cis and trans conformations themselves influence the permeability of the membrane, rather than the motion of isomerization doing so.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter