Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalysts That Are Less Precious
Researchers report progress on using iron and cobalt to replace precious metals in catalysts
by Stu Borman
December 16, 2013
| A version of this story appeared in
Volume 91, Issue 50

Precious metals such as iridium, rhodium, and ruthenium are widely used to catalyze chemical reactions, but “precious” means expensive, and for many years chemists have been searching for base metals that can catalyze reactions less expensively.
Successes have been few and far between. But three groups recently developed ways to use the less expensive metals iron and cobalt to catalyze hydrogenations—reactions used to synthesize pharmaceuticals, agrochemicals, and fine chemicals (Science 2013, DOI: 10.1126/science.1243550, 10.1126/science.1244466, and 10.1126/science.1242005).
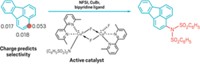
One way to create chiral compounds for such applications is asymmetric hydrogenation—hydrogenating an alkene carbon-carbon double bond enantioselectively, turning one carbon into a chiral center. Most catalysts for this reaction use precious metals. Paul J. Chirik and coworkers at Princeton University and collaborators at Merck Research Laboratories devised a high-throughput process to find base-metal versions.
The group selected sets of cobalt starting materials and chiral ligands and screened them to see which combinations worked best in solution to catalyze addition of molecular H2 to alkenes. They found a number of highly enantioselective cobalt-based hydrogenation catalysts, including one that “allows hydrogenation of two very different classes of alkenes,” a feat no single precious-metal catalyst can perform, Chirik says.
Catalysis mechanism specialist Peter H. M. Budzelaar of the University of Manitoba comments that the screening approach is promising for future catalyst discovery.
But the new catalysts’ turnovers—the number of reactions catalyzed before they stop working—are much lower than for precious-metal catalysts, and the ligands used, such as one called iPr-DUPhos, are so expensive that their cost would eclipse any savings on the type of metal used.
Therefore, catalysts reported in this initial study are unlikely to have industrial importance, comments catalysis specialist Per-Ola Norrby of the University of Gothenburg, in Sweden. “The study may be a step in the right direction, but there is a very long way to go yet.”
Chirik responds that similarly expensive ligands are currently used industrially and that the study’s findings “are more about new reactivity and opportunity than catalyst cost. This is only round one,” and future work should improve the catalysts.
In the second study, Robert H. Morris of the University of Toronto and coworkers report iron-based catalysts that convert ketones and imines to alcohols and amines enantioselectively by transferring hydrogen from 2-propanol. The group’s earlier studies of the mechanisms of such asymmetric transfer hydrogenations led them to believe that amine(imine)diphosphine ligands might activate iron to catalyze such reactions efficiently, and the new study validates that.
“They manage to get some spectacular results for imine hydrogenation, easily compatible with the best that can be achieved with a noble metal,” Norrby says. “The ligand is easy to make from relatively cheap starting materials, so this could well become a competitive process. From an industrial perspective, one drawback is that the stoichiometric reducing agent is not hydrogen but another alcohol. But in a synthesis lab this would be seen as an advantage since high-pressure hydrogen requires special equipment.”
In the third study, Matthias Beller of the University of Rostock, in Germany, and coworkers created nanoscale solid-surface-supported iron oxide-based catalysts that convert nitroarenes to anilines selectively—even in the presence of reducible groups other than aryl nitro groups, such as aldehydes, ketones, and carbon-carbon double bonds.
Catalysts made from noble metals can accelerate hydrogenations under mild temperature conditions, and other iron-based catalysts—such as the Haber-Bosch catalyst that converts dinitrogen to ammonia—can catalyze reduction reactions at high temperatures. But the Beller group’s catalysts use a less expensive metal and yet work under mild temperature conditions. Norrby says this type of catalyst “could well become the new standard in the field, both in synthesis labs and in industrial processes” for nitroarene-to-aniline reactions.
The three studies show it is difficult to hit the catalytic jackpot with base metals but illustrate the value of basic research and high-throughput screening in overcoming that barrier, Budzelaar says.
In a Science commentary, R. Morris Bullock, a catalysis specialist at Pacific Northwest National Laboratory, adds that the striking diversity of the three approaches shows “there is no exclusive single ‘recipe’ for success in developing catalysts based on cheap metals—an observation that is encouraging to chemists seeking to design and develop catalysts based on abundant, inexpensive metals.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter