Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Unexpected Uranium Chemistry Found In Contaminated Aquifer
ACS Meeting News: Results will aid remediation efforts
by Jyllian Kemsley
April 10, 2013

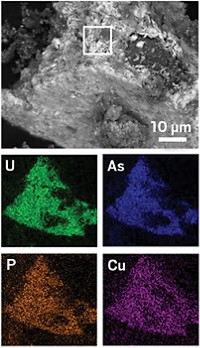
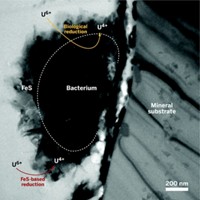
Cleaning up water in aquifers contaminated with uranium from processing ore for nuclear fuels could be carried out more effectively if the underground chemistry—how uranium converts between water-soluble U6+ and insoluble U4+—was well understood. Although scientists had believed that all U4+ in aquifers occurred as UO2, researchers report in a recent paper and at the American Chemical Society national meeting in New Orleans that a combination of microbial and abiotic reactions also produces other U4+ complexes (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1219198110).
More than a dozen former ore-processing sites in the western U.S. are contaminated with uranium. Cleaning up the sites is necessary to protect people, wildlife, and livestock from being exposed to uranium-contaminated groundwater.
One approach to treating uranium-contaminated aquifers is to add a microbial nutrient to create a bacterial bloom that consumes all of the oxygen and creates reducing conditions, say research team leaders John R. Bargar and Noémi Janot of SLAC National Accelerator Laboratory. After using this method on a uranium-contaminated aquifer in Rifle, Colo., the researchers found that the mechanism of U6+-to-U4+ reduction is complex. Iron sulfide minerals that deposit on biomass appear to reduce U6+ to U4+. Then, U4+ is not only converted to UO2, it also forms complexes with phosphate or other ligands from nearby biological material. The microbes themselves may also reduce U6+ to U4+.
The results will change how aquifer behavior is modeled, Bargar says. Right now, models assume that all U4+ turns into UO2.
Seeing the unexpected products indicates that scientists still have much to learn about uranium chemistry in natural systems, says Argonne National Laboratory’s Max Boyanov, who also studies subsurface mineral transformation.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter