Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Delivery
Bandages Silence Genes
RNA Interference: Nanocoatings on bandages and other medical materials could deliver RNAs capable of shutting off genes related to disease
by Katherine Bourzac
May 23, 2013

Medical researchers think specially tailored RNA sequences could turn off genes in patients’ cells to encourage wound healing or to kill tumor cells. Now researchers have developed a nanocoating for bandages that could deliver these fragile gene-silencing RNAs right where they’re needed (ACS Nano 2013, DOI: 10.1021/nn401011n). The team hopes to produce a bandage that shuts down genes standing in the way of healing in chronic wounds.
Small interfering RNAs, or siRNAs, derail expression of specific genes in cells by binding to other RNA molecules that contain the code for those genes. Biologists have developed siRNAs that target disease-related genes. But for these siRNAs to reach the clinic, researchers must find a way to deliver the molecules safely to the right cells. Unfortunately, free oligonucleotides like siRNAs don’t fare well inside the body or cells as enzymes and acids quickly chop them up, says Paula T. Hammond, a chemical engineer at Massachusetts Institute of Technology.
Other groups have tackled this delivery challenge by attaching siRNAs to chemical carriers that protect the oligonucleotides as they travel through the bloodstream. The pharmaceutical company Sanofi-Aventis asked Hammond to design a vehicle that would work at the site of a wound or tumor, releasing the siRNAs over a long period of time. The company hoped that putting the biomolecules right where they’re needed, without them having to survive a trip through the bloodstream, would increase the efficacy of the treatment.
Hammond and her colleagues produced an siRNA-containing nanocoating that could be applied to a wide range of medical materials, such as bandages or biodegradable polymers doctors could implant during surgery to prevent an excised tumor from coming back. As the coating slowly dissolves, it releases siRNA molecules tethered to protective nanoparticles.
The thin films consist of two different materials: a peptide called protamine sulfate and calcium phosphate nanoparticles decorated with the therapeutic siRNAs. Other researchers have shown that similar nanoparticles help the nucleotides evade destruction once they’re taken up by cells (J. Controlled Release 2010, DOI: 10.1016/j.jconrel.2009.11.008). The team alternately dips whatever they want to coat in water solutions of the two materials. The RNA and nanoparticles are negatively charged, and the peptides are positively charged. The two substances cling together due to electrostatic force, producing a film when the water dries.
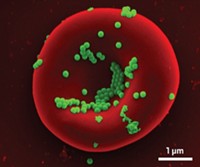
To test their delivery method, the researchers coated woven nylon bandages with 80-nm-thick films and applied the bandages to layers of human and animal cells in culture. In one experiment, a bandage loaded with 19 µg of siRNA per square centimeter released two-thirds of its load over 10 days. Other bandages made using siRNAs targeting the gene for fluorescent green protein almost completely shut down the protein’s production in cells expressing the gene. Hammond says the group is now testing bandages that knock down MMP9, a collagen-destroying protein associated with slow healing in chronic wounds.
Sarah C. Heilshorn, a materials scientist at Stanford University, thinks the coatings could reach the clinic rapidly because they use naturally derived materials that have already been approved by the Food and Drug Administration. Still the MIT team first must demonstrate that the coatings can turn off genes in animal models of wound healing, she says. Hammond says the group is currently wrapping up a series of such tests.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter