Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Measuring Cyanate In Sea Water For The First Time
Ocean Chemistry: Adapted technique measures low levels of cyanate in water, could allow researchers to study the ion’s role in the oceans’ nitrogen cycle
by Sarah Webb
June 14, 2013

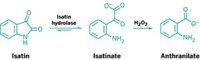
As researchers trace how nutrients cycle through ocean ecosystems, some groups have focused on cyanate (OCN-), a potentially important but difficult-to-measure source of nitrogen for aquatic microbes. Now researchers have reported a method that allows them to measure cyanate in seawater samples at nanomolar levels for the first time (Anal. Chem. 2013, DOI: 10.1021/ac400351c).
Phytoplankton drive more than half of the conversion of chemical elements into biological matter on Earth. But researchers don’t fully understand how nitrogen, a critical nutrient for the microbes, moves in and out of phytoplankton communities. Genetic research and laboratory studies have suggested that some types of phytoplankton use cyanate as a nitrogen source, says Brittany Widner, a graduate student at Old Dominion University. Widner and her adviser Margaret R. Mulholland wanted to study that process, but they didn’t have a way to measure cyanate concentrations in the water at low nanomolar concentrations.
Cyanate—like other reduced nitrogen sources in seawater, such as ammonium and urea— gets taken up quickly by organisms in marine environments, Mulholland says. This rapid cycling points to its potential importance to organisms but also makes the ion challenging to measure because it is so dilute in the water. Salt in sea water can also complicate cyanate measurements, Widner adds. For example, in ion chromatography, a peak for chloride ions can overshadow the one for the cyanate ion.
The researchers looked at other ways that scientists measure cyanate. They decided to adapt a fluorescence assay that biomedical researchers had developed for looking for cyanate in blood.
To test the method, Widner and her colleagues collected water samples in estuaries and in open waters. Back in the laboratory, they added 2-aminobenzoic acid to their samples. The compound reacted with cyanate present in the water to produce a highly fluorescent compound, 2,4-quinazolinedione. They then analyzed the reacted samples using high-performance liquid chromatography and looked for peaks for the fluorescent compound. By comparing peaks from their samples to those from a set of cyanate standards, they could calculate the cyanate levels in the samples. The detection limit for this method is 0.4 nM.
The work is “no small feat,” says Anton F. Post of the Marine Biological Laboratory, who discovered the gene for a cyanate processing enzyme in one type of cyanobacterium. The technique finally allows researchers to measure cyanate at levels that are relevant to the marine environment, he says.
The Old Dominion team now plans to use the method to examine cyanate concentrations in different locations and at different depths, as well as to measure the rate of cyanate uptake in organisms. As part of these experiments, Widner is headed out on a research vessel to the eastern tropical South Pacific Ocean this summer.
Further tweaks to the method may improve its sensitivity by another order of magnitude, the researchers say. With that detection limit, they expect that their technique could measure even the most dilute cyanate samples from open waters.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter