Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Novartis First-Generation Lung Cancer Drug Tweaked To Reduce Potential Side Effects
Medicinal Chemistry: Redesigned enzyme inhibitor designated as breakthrough therapy by FDA
by Louisa Dalton
June 20, 2013

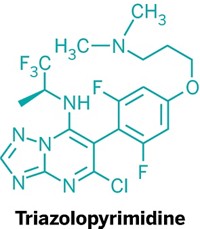
Certain lung cancer tumors express an enzyme called anaplastic lymphoma kinase (ALK), which is critical to the cancer’s survival and spread. As a result, ALK is a popular drug target. Now researchers describe how they redesigned an ALK inhibitor to avoid toxicity issues and produce a promising drug (J. Med. Chem. 2013, DOI: 10.1021/jm400402q). The new inhibitor, currently in clinical trials to treat non-small-cell lung cancer, is on fast-track review with the Food and Drug Administration.
In 2007, researchers at the Genomics Institute of the Novartis Research Foundation reported a potent ALK inhibitor (Proc. Nat. Acad. Sci. 2007, DOI: 10.1073/pnas.0609412103) that had “a serious liability,” says Pierre-Yves Michellys, director of medicinal chemistry at GNF. In the body, the compound’s electron-rich aniline group tends to change into a highly reactive quinone that can bind to nontarget proteins. Michellys and his colleagues determined that the key to this reactivity lies with a nitrogen atom connected to the aniline ring.
So they designed a set of variations on the compound with the nitrogen in a different position. These new drug contenders lacked the tendency to react with proteins. One variation, LDK378, turned out to be extremely selective for ALK. In rats injected with ALK-positive cancers, the compound significantly shrunk or even totally eliminated tumors.
The FDA recently designated LDK378 as a breakthrough therapy based on encouraging results from early clinical trials in patients with ALK-positive, non-small-cell lung cancer.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter