Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Heated Nanoparticles Throw Gene Therapy Switch
Synthetic Biology: Magnetic nanoparticles heat up and turn on synthetic genes inserted into cancer cells
by Melissae Fellet
November 11, 2013

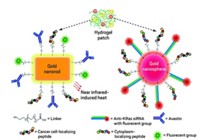
In a strategy known as gene therapy, scientists insert engineered DNA into diseased cells in order to treat or kill them. Now, researchers have combined nanotechnology and synthetic biology to create a simple switch to turn on such genes inside cells. They demonstrate that heat generated by magnetic nanoparticles activates the engineered genes, slowing tumor growth in mice (ACS Synth. Biol. 2013, DOI: 10.1021/sb4000838).
For gene therapy to reach clinical applications, such as for treating cancer, researchers need to activate their engineered gene sequences only in the diseased cells. That’s because in the course of introducing the synthetic genes, some healthy cells also may pick up the DNA packages. So to prevent activating synthetic genes in healthy cells, researchers want to design genetic circuits that can be triggered selectively.
Currently, there are only a few ways to do that, typically through applying drugs to the cells. Masamichi Kamihira of Kyushu University, in Japan, and his colleagues thought magnetic fields would be a more useful trigger. The fields travel deep into tissues and can be targeted to certain areas of tissue to avoid turning on genes in healthy cells.
Researchers have long known that nanoparticles made from magnetite generate heat when placed in an alternating magnetic field. So Kamihira and colleagues engineered a series of genes controlled by a heat-activated promoter. Promoter regions are stretches of DNA that help initiate transcription of genes nearby. Some promoters require external stimuli, such as heat, to launch the transcription machinery.
Kamihira and his team connected a few genes to this promoter. One codes for tumor necrosis factor-α (TNF-α), a protein that induces cell death. Another gene codes for a protein that activates a second element in the circuit. This element keeps transcription of TNF-α going long after the heat stimulus is gone. So after a pulse of external heat, a positive feedback loop turns on to continuously produce the cell-killing protein.
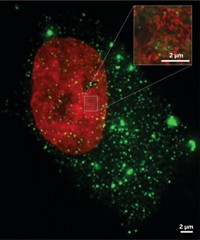
To test the nanoparticle-gene circuit combination, the researchers inserted the promoter and genes into human lung cancer cells. They encased the DNA inside liposomes that fuse with the cells’ membranes and release the genetic information into the cell, where it can find the cell’s transcription machinery. Then they added magnetite nanoparticles coated in lipids, which also slipped inside the cells. When the researchers applied an alternating magnetic field for 30 minutes, the cells warmed to 43 °C, and 90% of them died. Heat from the nanoparticles alone also killed the cells, but not as effectively as when they were combined with the heat-triggered gene circuit. In fact, when the team examined cells containing the nanoparticles and a plasmid without any of the therapeutic genes four days later, only 60% of the cells exposed to the magnetic field were dead.
The researchers also tested their method in mice with tumors grown from injected human lung cancer cells. They also injected the gene-containing liposomes and magnetic particles into the tumor site. Some animals were exposed to an alternating magnetic field for 30 minutes; other mice were not. After 30 days, mice placed in the magnetic field had tumors that were just 8% of the volume of those in unexposed mice.
Cheemeng Tan at the University of California, Davis, says that combining nanoparticles and engineered genes for anti-cancer treatment is a new direction for synthetic biology that could increase the specificity and efficacy of treatment. But this method still faces the same two challenges that other cancer gene therapies must overcome, he says: The researchers must ensure that adequate amounts of the engineered circuits get into cancer cells and that the treatment kills only cancer cells, not healthy ones.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter