Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Silicone Chip Recreates Cancer’s Microenvironment
Microfluidics: A device for high-throughput anticancer drug screening more accurately simulates the blood circulation system bordering a tumor
by Louisa Dalton
March 19, 2014

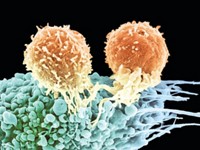
Killing cancer cells growing on a petri dish is an entirely different beast than fighting cancer cells in the human body. To provide a more realistic platform for screening anticancer drugs, bioengineers have created a device that better replicates the environment around human tumors than conventional cell culture does. They designed a microfluidic chip that imitates the blood circulation system alongside a three-dimensional tumor (Anal. Chem. 2014, DOI: 10.1021/ac403899j). With further development, the chip should be useful for both high-throughput drug screening and for helping doctors quickly pinpoint the best cancer therapy for individual patients.
When pharmaceutical companies screen drugs for anticancer activity, they do it on a large scale. Robotic pipettes administer thousands of drugs at a time into individual wells of cultured cancer cells. But a flat layer of cells is not a good simulation of cancer in the human body, says Sihong Wang of the City College of New York. “Somehow,” she says, “we have to go to 3-D cell culture and still keep the ability to do high-throughput screening.”
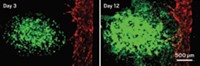
Wang and her coworkers conceived a three-layer microfluidic device. In microchambers carved into the bottom layer of a silicone chip, the researchers culture cancer cells in a 3-D hydrogel to recreate a solid tumor. In the top layer, they mimic a small blood vessel running next to the cancer cells with a microchannel carpeted with endothelial cells. The middle layer allows diffusion between the top and bottom layer via clustered pores.
To test the device, the scientists seeded human breast cancer cells encapsulated in the hydrogel in the lower microchambers and spread a layer of human endothelial cells in the top microchannel. When they flowed cell culture medium through the simulated blood vessel, the cancer cells remained healthy for more than two weeks.
The researchers designed the chip’s microchannel to prevent the cancer cells from experiencing the forceful tug of the fluid flow, as happens in two-dimensional cell culture. That shear stress could kill the cells or alter their response to drugs, Wang says. However, the microchannel still allows diffusion of nutrients and waste, like a blood vessel does.
When they flowed anticancer drugs through the simulated vessel, they found the cancer cells were more resistant to apoptosis than cells cultured in a device without an endothelial-cell lining. The scientists suspect the endothelial cells in the chip delay delivery of the drug to the cancer cells and thus create an environment more like the human body.
Wang is exploring the possibility of using the chip for clinical decision-making. A physician could insert a patient’s own biopsied cancer cells in the chip, test 20 drugs at a time, and quickly narrow down the patient’s drug choices to the most effective treatment.
For drug firms, Wang and her coworkers are engineering a second-generation chip with microchannels oriented to flow across the chambers of cancer cells so that it can simultaneously test up to 100 drugs and 20 cancer cell lines.
John W. Haycock of the University of Sheffield says this type of chip, with two types of cells situated in space as they might be in the body, is “more advanced than any 2-D culture system.” For anticancer drug screening, scientists could use the data from a 2-D culture, then a 3-D test, to focus and reduce the need for live animal testing, he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter