Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Carbon Dots For White LEDs Made In One Step
Nanotechnology: Simple, low-cost process yields carbon nanoparticles that emit bright white light
by Prachi Patel
April 8, 2014

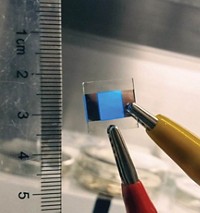
Chemical engineers report an easy, low-cost method to make carbon nanoparticles that glow pure white (Ind. Eng. Chem. Res. 2014, DOI: 10.1021/ie500602n). The team used the particles, known as carbon dots, to make white light-emitting diodes. These LEDs could be an alternative to devices based on quantum dots containing toxic metals, the researchers say.
Quantum dots, which are semiconducting nanocrystals, are a promising technology for LEDs used in lighting and displays. The materials emit a pure, saturated color that can be tuned simply by changing the size of the dots. Scientists usually make quantum dots with compounds that contain toxic heavy metals such as cadmium and lead. They’ve used these nanoparticles to fabricate LEDs of different colors, including white ones.
In 2006, researchers proposed fluorescent carbon nanoparticles as a nontoxic alternative to traditional quantum dots (J. Am. Chem. Soc., DOI: 10.1021/ja062677d). Scientists have come up with various approaches for making LEDs with these carbon dots. But the methods are expensive and complex, and require toxic starting materials, says Su Chen, a chemical engineer at Nanjing University of Technology, in China. Also, Chen says, most carbon dot LEDs just emit dull blue light.
Chen, Cai-Feng Wang, and their colleagues wanted to develop a simple, economical process for making carbon dots that glowed purely white. They came up with a one-step method: heating a mixture of polyacrylic acid and glycerol at 230 °C for two hours. After that, the team spins the product at high speed to remove any large carbon particles and then filters out excess glycerol. The resulting solution contains nearly spherical carbon nanoparticles that are about 3.4 nm wide.
The carbon dot solution appears brownish yellow in sunlight but emits a bright white light when the researchers shine ultraviolet light on it. The light intensity did not change much after 30 hours of high-power UV irradiation, or after storing the carbon dots for three months. Such stability is crucial for practical applications, Chen says.
The researchers made white LEDs with the carbon particles using a standard design. They applied a coating of the dots on top of an LED chip that emits ultraviolet light; the dots glow white when excited by these wavelengths. The team reports that the light was comparable to the color emitted by commercial white LEDs.
“This is very impressive work,” says Chengde Mao, a chemist at Purdue University. The stability and water-solubility of the reported carbon dots also make them a great choice for imaging cells and biological tissues, he says.
This work demonstrates the possibility of using carbon dots for lighting, says Yuwu Chi, a chemist at Fuzhou University, in China. “Although many blue, green, and yellow fluorescence carbon dots have been synthesized and applied as sensing materials, the application of white fluorescent carbon dots in LEDs is seldom reported,” he says. Polyacrylic acid as a raw material makes the method attractive, because it is made industrially on a large scale, he adds. “The synthesis method is simple, green, and of low cost.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter