Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Multifaceted Collaboration Solves Natural Product Puzzle
Biosynthesis coupled with computational studies unravel the origins of a set of bioactive fungal alkaloids
by Carmen Drahl
May 14, 2014

Chemists have yet to learn all of the molecule-making tactics of the family of fungi that makes penicillin. And as a research report shows, collaborative efforts to further unravel fungal biosynthetic pathways can help scientists find new antibacterial and antitumor agents (Nature 2014, DOI: 10.1038/nature13273).
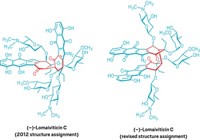
Richmond Sarpong and his group at the University of California, Berkeley, had run into a structure elucidation problem along one of these biosynthetic pathways that left them scratching their heads and issuing a call for help. On the basis of work from other teams, they expected that the alkaloids citrinalin B and cyclopiamine B would be pseudoenantiomers—that is, nearly mirror images of one another, but not quite. Yet that’s not what the reported structures for the molecules indicated.
The Berkeley team proposed a new structure for citrinalin B and asked UC Davis computational chemist Dean J. Tantillo for backup. The calculations supported the idea that the originally reported structure of citrinalin B had been misassigned. Armed with that evidence, Sarpong’s lab collaborated with Yale University’s Scott J. Miller to carry out chemical syntheses that confirmed the revised structure.
“Most synthetic chemists would have stopped at this stage,” organic chemist John L. Wood of Baylor University writes in a Nature commentary. “Sarpong chose to take things further.”
With Roberto G. S. Berlinck of the University of São Paulo, in Brazil, the chemist who first isolated the citrinalin natural products, Sarpong’s team carried out additional biosynthesis studies. Those results support a long-held idea that citrinalin, cyclopiamine, and related molecules come from a precursor containing a bicyclo[2.2.2]diazaoctane. What’s more, they discovered a new molecule, citrinalin C, which they think may be such a precursor.
This work, Wood adds, “beautifully illustrates how natural products continue to inspire research in synthesis, and that these efforts can benefit greatly from collaboration.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter