Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Lowering The Cost Of Carbon Capture
ACS Meeting News: Phase-changing ionic-liquid-based material could reduce the energy needed to trap CO2
by Jyllian Kemsley
August 14, 2014

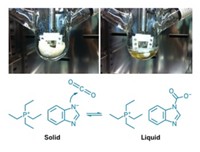
An ionic-liquid-based material that changes from solid to liquid upon reacting with carbon dioxide could lower the energy costs of capturing carbon dioxide from power plant emissions.
Joan F. Brennecke, professor of chemical and biochemical engineering at the University of Notre Dame, discussed the phase-change ionic liquid (PCIL) chemistry at The Kavli Foundation Innovations in Chemistry Lecture at the American Chemical Society national meeting this week in San Francisco.
The PCIL is composed of a tetraethylphosphonium salt that is solid at normal postcombustion flue-gas temperatures of 40 to 80 °C. CO2 reacts stoichiometrically and reversibly with an aprotic heterocyclic anion, reducing the melting point of the material and turning the solid into a liquid (Energy Fuels 2014, DOI: 10.1021/ef501374x).
In the proposed process, a slurry of the solid PCIL and PCIL-CO2 would be used in an absorber to capture more CO2. The resulting liquid would be transferred to a spray dryer, where it would be heated to drive off the CO2 for storage elsewhere while the PCIL is reused.
The combined enthalpies of the CO2 binding and release reactions and the associated phase changes require less external energy than existing carbon-capture systems. Those based on aqueous amine chemistry use 28% of the energy generated by a conventional coal power plant and boost electricity costs by 6 cents per kilowatt hour. The PCIL approach would consume about 23% of the energy generated and increase electricity costs by just 4.1 cents per kilowatt-hour, Brennecke said.
The phase-change concept is “clever and innovative,” says Robert J. Perry, a senior chemist at GE Global Research. He cautions that the approach has only been demonstrated in the lab and needs further study to determine its viability at larger scale.
PCILs may also have applications other than CO2 capture, such as in heat storage and chemical separations, in which CO2 could perhaps be used to trigger a phase change to trap a particular substance, says James H. Davis Jr., a chemistry professor at the University of South Alabama.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter