Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Rhodium Expands Collection Of Metal-Based Anticancer Agents
ACS Meeting News: New class of inorganic reagents may provide less toxic and more versatile alternatives to popular platinum-based drug
by Stephen K. Ritter
August 13, 2014
More than half of all cancer patients receive a platinum-based drug such as cisplatin as part of their chemotherapy. But the compounds have dangerous side effects and many tumors develop resistance to the drugs, prompting chemists to seek out less toxic and more selective alternatives.
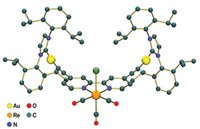
In one of the latest examples, Amanda David, a graduate student in chemistry professor Kim R. Dunbar’s group at Texas A&M University, described a family of promising dirhodium complexes during a symposium on bioinorganic chemistry sponsored by the Division of Inorganic Chemistry at the American Chemical Society meeting in San Francisco this week.
The Texas A&M team developed the dirhodium complexes with Claudia Turro at Ohio State University. In cell culture experiments, the compounds exhibited lower toxicities and were as effective or better than cisplatin against lung cancer cells (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja503774m).
To track the movement of their rhodium complexes in vivo, the researchers have equipped the rhodium compounds with light-absorbing dye ligands. The dye complexes are inactive toward cancer cells in the dark, David reported, but when lit up they kill nearby cancer cells. This property could make them useful for imaging tumors and for treating skin cancer and endoscopically accessible tumors. The team previously reported ruthenium complexes with similar properties that are highly toxic to cervical cancer cells (Organometallics 2014, DOI: 10.1021/om500001h)
The Food & Drug Administration’s 1978 approval of cisplatin was a defining moment in modern medicine, Dunbar said. Since then it has saved the lives of many people, particularly testicular and ovarian cancer patients. But the damage it does to the kidneys and other organs and its low specificity for cancer cells remain troubling drawbacks. Indeed, only three metal-based anticancer drugs—all platinum compounds—have ever been approved in the U.S. But a handful of low-toxicity ruthenium compounds being developed by other researchers are now in clinical trials, and Dunbar thinks the rhodium and ruthenium complexes being developed in her group “are looking more and more promising as potential drug candidates.”
“It’s an exciting time to be working in the field of medicinal inorganic chemistry of anticancer agents” commented Edith C. (Phoebe) Glazer of the University of Kentucky, whose group is developing light-activated ruthenium anticancer agents. The Texas A&M team’s compounds localize to the mitochondria of cancer cells. That observation might help scientists better understand the compounds’ mechanism of action and predict and avoid undesired side effects, Glazer told C&EN.
“A major issue in medicinal inorganic chemistry is the misconception that all metal-based compounds are systemically toxic,” Glazer explained. “The toxicities of inorganic agents, such as cisplatin, are due to their mechanism of action, the same as for organic drugs or biological agents, not to some intrinsic properties of heavy metals. The increasing interest in defining the molecular and cellular effects of cytotoxic inorganic agents should improve confidence in translating compounds from basic science research into preclinical models.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter