Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Trifluoromethanide Anion Outlives Expectations
Believed to be a transient species, a key intermediate in fluorine chemistry has been captured in a stable form for the first time
by Stephen K. Ritter
September 3, 2014

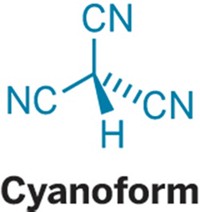
For chemists, adding fluoroalkyl groups to molecules is an important way of controlling properties, such as the bioactivity of drugs and the performance of plastics and electronic materials. The trifluoromethanide anion, CF³–, has long been the proposed key intermediate in these reactions, except that no one has been able to trap the anion—it’s been thought to only pop in and out of existence.
Researchers at the University of Southern California now report capturing and studying a stable trifluoromethanide complex for the first time. The finding brings added clarity to trifluoromethylation reactions and is expected to help guide research and commercial developments.
“This study not only dispels the myth that the CF³– anion is a fleeting intermediate in nucleophilic trifluoromethylation chemistry, but it also serves as a valuable platform for future mechanistic studies in this exciting research field,” comments organofluorine chemist Ryan Gilmour of the University of Münster, in Germany.
Chemists have suspected that CF³– undergoes fast, reversible defluorination to form difluorocarbene and fluoride as part of its reactivity. But the USC researchers—including G. K. Surya Prakash, Karl O. Christe, and George A. Olah—discovered through thermodynamics calculations that the anion should be persistent at low temperature.
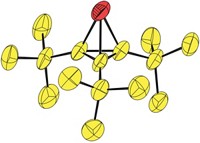
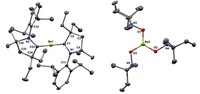
They then determined that the optimal way to stabilize trifluoromethanide would be to pair it with the right metal cation. Potassium topped the list, and the team proceeded to capture the anion in bulk amounts as a potassium crown ether complex and confirmed its existence through low-temperature NMR spectroscopy. The anion remains stable in solution for several days at –78 °C (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201406505).
The USC team says the anion’s role in trifluoromethylation reactions is directly evident from the potassium complex’s reactivity with an array of electrophilic reagents. For example, the researchers show that treating the complex with excess CO2 leads to potassium trifluoroacetate. They believe previous failures to isolate the intermediate can be attributed to its low thermal stability, which stems from its highly nucleophilic nature (strong basicity).
“A lot of experts didn’t think this anion could be isolated,” says fluorine chemist Tobias Ritter of Harvard University. “So this discovery teaches us a lot of fundamental knowledge, and it teaches us—once again—that we should not believe all ‘rules’ that spook around in this field.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter