Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Water’s Role In Key Oxidation Revealed
Catalysis: Water found to provide active sites for carbon monoxide oxidation on catalytic surface
by Stu Borman
September 5, 2014

The detailed molecular steps that occur when carbon monoxide is oxidized to carbon dioxide on a catalytic gold-titanium dioxide surface have for years been uncertain and controversial, despite intense study.
A collaborative team in Texas has used a combination of experiment and theory to determine, for the first time, exactly how the reaction works and the key role water plays in it (Science 2014, DOI: 10.1126/science.1256018). The findings help clarify earlier but less comprehensive work, and they could advance prospects for the proposed commercial use of the reaction to selectively oxidize CO in CO-H2 mixtures produced industrially.
Small amounts of CO are often found to be mixed with hydrogen when it is made from natural gas and light petroleum, the major route to industrial H2 production. And CO acts as a contaminant or poison in some end uses of H2, such as in fuel cells. An efficient way to remove CO from H2 in these mixtures is needed, and oxidizing CO to CO2 over Au/TiO2 can potentially do the job.
But the activity and selectivity of the reaction would need to be optimized for industrial use. A molecular-level understanding of the process would help make this more feasible, but it had not yet been achieved. For example, water was known to have a major influence on the reaction, but exactly how was unknown.
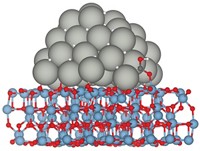
The new work by Bert D. Chandler of Trinity University, in San Antonio; Lars C. Grabow of the University of Houston; and coworkers solves that mystery. They find that water adsorbed at the Au/TiO2 interface creates active sites for the reaction.
The Trinity group used kinetics and infrared spectroscopy experiments to show that a proton transfer associated with water weakly adsorbed to the Au/TiO2 surface was involved in a key mechanistic step. And the Houston team used density functional theory simulations to reveal that the protons from water join with oxygen molecules on the surface to form Au–OOH species that react with Au-bound CO. The reaction produces Au–COOH, which resolves into Au, CO2, and water.
“This paper presents long-sought answers on the roles of water and the metal-support interface in CO oxidation over supported Au catalysts,” comments CO oxidation specialist Harold H. Kung of Northwestern University. “It appears to have resolved both issues with a unifying explanation.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter