Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Protons Pass Through The Notoriously Impermeable Material Graphene
Materials: Membranes made of this and other two-dimensional materials could improve hydrogen fuel cells, which require a proton-conducting barrier
by Matt Davenport
December 1, 2014

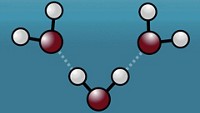
Graphene and atomically thin crystals of boron nitride conduct protons far better than predicted and could radically enhance the performance of hydrogen fuel cells, according to an international research team (Nature 2014, DOI: 10.1038/nature14015).
Over the past decade, scientists have found that forcing ions or atoms through even a single layer of graphene is virtually impossible without the help of a particle accelerator. Theorists had predicted that, at room temperature, hydrogen could take billions of years to cross a graphene monolayer. This meant graphene, and likely other two-dimensional crystals, would probably make for awful proton-conducting membranes, essential ingredients in certain hydrogen fuel cells.
But researchers led by Andre K. Geim of the University of Manchester, in England, have now experimentally demonstrated that pristine single layers of graphene and hexagonal boron nitride (hBN) conduct protons surprisingly well. At temperatures more than about 100 °C, the membranes achieve conductivities that make them attractive for use in fuel cells, Geim says, and unlike other proton-conducting materials, these membranes are impermeable to other ions and atoms.
In the context of hydrogen fuel cells, an ideal proton-exchange membrane separates a compartment containing a hydrogen fuel source from another chamber filled with an oxidant, passing only protons between the two. Current real-world membranes permit some mingling between fuel and oxidant, which saps device performance.
Graphene and hBN membranes could reduce this chemical crossover by orders of magnitude, says Geim, who won a share of the 2010 Nobel Prize in Physics for his pioneering work with graphene.
Scaling up the production of such membranes for commercial fuel cells could be a challenge, but researchers are already making graphene sheets on the square-meter scale, says Rohit N. Karnik of Massachusetts Institute of Technology, a mechanical engineer who was not involved with the study. He adds that he is struck by how reproducible and conclusive the new results are. “I am really impressed with the quality of this work,” he says.
Geim and his team have yet to work out exactly how protons permeate the 2-D membranes, although they know that the density of the electron cloud between the membranes’ atoms plays a role.
At room temperature, hBN membranes conduct protons better than graphene, the researchers found. Nitrogen atoms within an hBN network attract valence electrons more strongly than do the boron atoms within the network. The interstitial electron cloud within hBN becomes thin as a result. The symmetric arrangement of graphene’s carbon atoms creates a more uniform, dense electron cloud that is tougher for protons to penetrate.
The team also studied 2-D molybdenum disulfide membranes, which had the densest electron clouds according to the team’s simulations and the poorest proton conductivity according to their measurements.
Geim hopes theorists will help solve the remaining mysteries of proton transport, such as why graphene outperforms hBN at elevated temperatures. For now, he says, this study emphasizes the importance of experimental science. Without it, no one would know just how easily protons move through graphene, he adds.
According to Geim, “If you read too much theory before doing experiments, you might miss new phenomena.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter