Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Best Effort Yet To Make Direct Methane Fuel Cell A Reality
Energy: Catalyst-embedded electrode helps cell generate more power than previous attempts to convert methane directly to electricity
by Stu Borman
October 29, 2015
| A version of this story appeared in
Volume 93, Issue 43
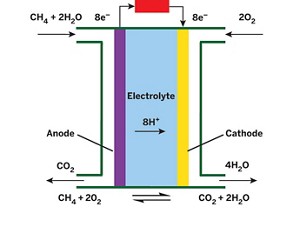
A new device uses a platinum catalyst to break down methane to produce electricity at low temperatures, generating more power than any previous direct methane fuel cell.
Currently, methane, a primary constituent of natural gas, is burned to produce heat and other forms of energy for homes and businesses. Direct methane fuel cells—in which methane is used directly, without first being converted into hydrogen—could produce energy more efficiently than combustion-based methods.
But such fuel cells must break methane’s strong C–H bonds, and that generally requires high operating temperatures between 650 and 1,100 °C, which add significantly to a device’s operating costs. Researchers developed a low-temperature direct methane fuel cell in 1962, and Andrew M. Herring of Colorado School of Mines and coworkers revived the concept in 2012. But both designs generated negligible amounts of power.
Now Herring and Brian G. Trewyn at Colorado School of Mines, T. Brent Gunnoe of the University of Virginia, and coworkers have used an organometallic complex to activate methane C–H bonds in a low-temperature direct methane fuel cell (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b06392).
The team covalently attached a low-temperature methane-activating platinum catalyst to conductive carbon and used the material as a fuel-cell anode. The material catalyzes an oxidation process that releases carbon dioxide, electrons, and protons. The protons then react with oxygen at the cathode to produce water.
The process converts methane directly to electricity at 80 °C and generates five times the power of any previous low-temperature direct methane fuel cell. But it has limitations: The catalyst deactivates over time, diffusion of methane into the anode is limited, and maximum power density is still too low for real-world use.
The approach demonstrates feasibility, “and there is ample room for improvement,” comments catalysis specialist Matteo Cargnello of Stanford University. “It is an elegant and novel concept that might open new perspectives in the efficient and clean use of natural gas,” adds materials scientist Paolo Fornasiero of the University of Trieste, Italy.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter