Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
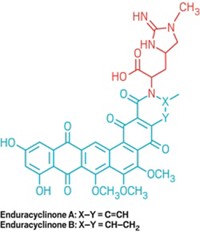
Biological Chemistry
Newfound Antibiotic Is Gargantuan And Unfortunately Deadly
Natural Products: Large macrolactone features a 52-membered ring and overall contains 105 carbon atoms, of which nearly half are chiral centers
by Stephen K. Ritter
March 12, 2015
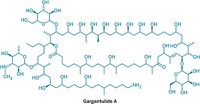
Gargantulide A is a big, utterly complex, and fantastic molecule to gaze upon. But the polyketide natural product with promising antibiotic properties is so deadly toxic it will have to be shelved (Org. Lett. 2015, DOI: 10.1021/acs.orglett.5b00068).
An international research team led by William H. Gerwick of the University of California, San Diego, and Mark S. Butler, formerly of MerLion Pharmaceuticals, in Singapore, found gargantulide A in a high-throughput screen of the biochemical effluent of a Streptomyces bacterium. That was the easy part.
More challenging was the team’s effort to elucidate the structure of gargantulide A, which required implementing nearly every conceivable NMR spectroscopy analysis method. The molecule features a 52-membered macrolactone ring and overall contains 105 carbon atoms, of which nearly half are chiral centers. Gerwick praises UCSD team member Jung-Rae Rho’s NMR prowess.
The researchers found that gargantulide A kills pathogenic bacteria such as MRSA (Staphylococcus aureus) and Clostridium difficile. But injecting mice with the compound led to a quick death for the animals. The severe toxicity has precluded any further development of the compound as an antibiotic.
Gargantulide A is “a pretty amazing molecule,” Gerwick says. “Nature still has some surprises for us in terms of novel structures with powerful biological properties.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter