Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Proteins Nitrated At Specific Sites
ACS Meeting News: Approach could enable study of the role of α-synuclein nitration in Parkinson’s disease
by Stu Borman
March 25, 2015

Researchers have developed the first synthetic method to make proteins with nitrated versions of tyrosine in specific sites. The method may make it easier to study how α-synuclein, a protein implicated in Parkinson’s disease, might undergo alterations that then help bring about the disabling condition.
Specifically, the researchers used the technique to study the effects of replacing tyrosine with 3-nitrotyrosine in α-synuclein, which can aggregate to form fibrils found in the brains of Parkinson’s disease patients.
α-Synuclein has four tyrosine residues. These are nitrated to 3-nitrotyrosine in some endogenous α-synucleins and in most Parkinson’s disease aggregates. Researchers believe tyrosine nitration significantly alters α-synuclein’s properties and may lead to neurodegeneration, possibly by promoting α-synuclein oligomerization and fibril formation.
But it has been difficult to substitute 3-nitrotyrosines site-specifically into native α-synuclein to study how individual or multiple substitutions might help cause Parkinson’s disease. The new technique solves that problem.
Until now, researchers have studied α-synuclein nitration by using chemical agents that nitrate the protein randomly; by mutating one or more tyrosines to phenylalanines to knock out nitration at selective sites; and by using unnatural amino acid mutagenesis to replace a specific tyrosine with 3-nitrotyrosine. But nitrating agents create heterogeneous product mixtures; mutating tyrosines to nonnative phenylalanines alters α-synuclein’s properties in unpredictable ways; and unnatural amino acid mutagenesis has low yields and can’t easily handle more than a single substitution.
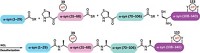
In a symposium sponsored by the Physical Chemistry Division at the American Chemical Society national meeting in Denver, Hilal A. Lashuel and coworkers at the Swiss Federal Institute of Technology (ETH), in Lausanne, reported their technique to nitrate one or more tyrosines in α-synuclein site-specifically. They used solid-phase peptide synthesis to make α-synuclein fragments in which 3-nitrotyrosines are substituted for tyrosines as needed. This allowed the team to nitrate one or more tyrosines without affecting any of the others. They then used native chemical ligation to combine the fragments in the proper order and desulfurized the resulting protein using a method devised by coworker Ritwik Burai. This modified desulfurization process prevents nitro-group reduction, a side effect when conventional desulfurization is used to terminate native chemical ligation.
The researchers also reported their work in the Journal of the American Chemical Society this year (DOI: 10.1021/ja5131726).
With the new technique, Lashuel and coworkers studied some of the effects of tyrosine nitration on α-synuclein and how the corresponding structural changes affected oligomerization and fibril formation in vitro.
Vladimir Uversky of the University of South Florida, who investigates α-synuclein nitration, commented that the nitrated α-synucleins produced by the technique will now make it possible “to investigate in great detail the role of site-specific nitration in regulating the structure, function, oligomerization, and aggregation of this enigmatic protein in health and disease.”
The approach “will facilitate the development of antibodies and imaging agents for the detection and quantification of different α-synuclein species and aggregates” that form as the disease progresses, Lashuel said. His team now plans further studies of such effects in Parkinson’s cell and animal models.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter