Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
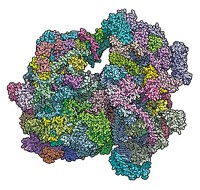
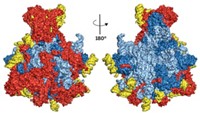
First Pictures Of The Mitoribosome Taken
Biomolecular Structure: Researchers obtain near-atomic-level views of the complete human and pig mitochondrial ribosomes
by Stu Borman
April 3, 2015

Structures of complete ribosomes from mammalian mitochondria at near-atomic resolution have been obtained for the first time by two research groups in Europe. The structures represent a landmark achievement in biomolecular research, opening a window on mitochondrial mutations and dysfunction and perhaps a path to new medicines.
Ribosomes translate genetically transcribed mRNA templates to synthesize cellular proteins. Most mammalian ribosomes are found in the cell cytoplasm, but mitochondria, the energy-producing organelles of cells, have their own ribosomes. These so-called mitoribosomes synthesize only 13 proteins in mammalian cells, leaving the rest of the protein synthesis workload to their counterparts in the cytoplasm.
Research teams had previously obtained X-ray crystal structures of complete bacterial and eukaryotic ribosomes in the cell cytoplasm. But the mitoribosome—which, like the cytoplasmic ribosome, has two subunits, one large and one small—has proved to be a tougher nut to crack. This is because it occurs in smaller numbers and is harder to purify. Its small subunit is also highly flexible, moving around a lot when having its picture taken.
Researchers structurally analyzed the mitoribosome’s large subunit in earlier work. Now, some of the same scientists used cross-linking and other tricks to get the small subunit to behave so they could obtain cryo-electron microscopy structures of the complete mitoribosome. Venki Ramakrishnan and coworkers at the Medical Research Council Laboratory of Molecular Biology, in Cambridge, England, determined the structure of the human mitoribosome, including 80 proteins and three ribosomal RNAs, at 3.5-Å resolution (Science 2015, DOI: 10.1126/science.aaa1193). And Nenad Ban of the Swiss Federal Institute of Technology (ETH), Zurich and coworkers determined the 3.8-Å structure of the pig mitoribosome with an mRNA and two tRNAs bound (Science 2015, DOI: 10.1126/science.aaa3872).
Such high levels of detail could at one time be achieved only with X-ray crystallography, but in recent years, cryo-electron microscopy has improved so much that it can now be used to obtain structures at 4-Å resolution or better. Atomic resolution is considered to be about 3 Å.
The new structures will help researchers probe how mitoribosome mutations cause mitochondrial dysfunction and will provide “a third leg to the stool” in terms of antibiotic development, comments protein biosynthesis expert Jamie H. D. Cate of the University of California, Berkeley. Some antibiotics, such as aminoglycosides, target the bacterial ribosome but have severe side effects stemming in part from interactions with human mitoribosomes. “Having the mitoribosome structures to look at will help scientists understand why some classes of antibiotics are toxic” and perhaps help them develop less toxic versions, Cate says.
Ribosome specialist Rajendra K. Agrawal of the Wadsworth Center, in Albany, N.Y., notes that further structural studies will be needed to develop detailed molecular models of various steps of mitochondrial translation, but “the new structures are major leaps toward understanding the molecular details of this process.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter