Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News: Bimetallic Clusters Exhibit Exceptional Catalytic Ability
by Mitch Jacoby
August 17, 2015
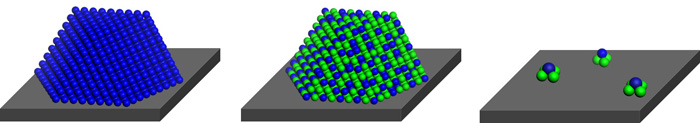
Minuscule metal clusters consisting of just a few atoms of two types of metals can catalyze chemical reactions with extraordinary selectivity if the clusters are supported on a solid and kept isolated from one another.
Those findings, presented Sunday at the American Chemical Society national meeting in Boston, suggest ways to prepare highly effective industrial catalysts from tiny amounts of costly metals.
To prepare the catalytic clusters, Franklin (Feng) Tao of the University of Kansas, Lawrence, and coworkers first synthesized cobalt oxide nanorods, which served as the solid support. Then by using a precipitation method, they deposited Rh3+ species on the nanorod surfaces and followed that with oxidation and reduction steps. High-resolution microscopy and other analytical methods confirmed that the synthesis yielded clusters of RhCo3 uniformly dispersed on the rods.
Then the Kansas team evaluated the clusters’ knack for catalyzing the reduction of NO in the presence of CO to form N2 and CO2. This reaction takes place in some automobiles’ catalytic converters to clean up the vehicles’ emissions. At a symposium organized by the Division of Catalysis Science & Technology, Tao reported that the RhCo3 clusters were highly active and 100% selective in converting NO to N2 at temperatures as low as 110 °C. The clusters also outperformed rhodium-cobalt alloy nanoparticles: Depending on how the particles were supported, they were either inactive as catalysts below 250 °C or they converted NO to an unwanted mixture of N2 and N2O.
Using quantum calculations, the group showed that the difference between Rh-Co clusters and nanoparticles arises from the unique cationic nature of the clusters and the stronger bonds they form with reaction intermediates.
Hong Yang, a professor of chemical engineering at the University of Illinois, Urbana-Champaign, remarks that there has been an exciting development in catalysis in recent years in which researchers have shown that individual atoms can function as active catalytic sites. Tao and colleagues have expanded this line of research, he says, from a single atom to a single RhCo3 cluster, “which shows exceptional selectivity in reducing NO exclusively to N2.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter