Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Aldolase enzyme gets a makeover
Changing just two amino acids broadens the catalytic protein’s range for aldol condensations
by Mark Peplow, special to C&EN
March 7, 2016
| A version of this story appeared in
Volume 94, Issue 10
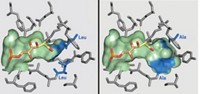
To be used industrially, an enzyme usually needs to undergo some surgery, for instance to resculpt its active site into a shape that will accept a range of chemical substrates. But the alterations that allow an enzyme to be more promiscuous can cripple its chemical activity. Researchers have now given the active site of an Escherichia coli aldolase a minimal makeover that enables it to handle a wider range of substrates without a loss in performance (ACS Catal. 2016, DOI: 10.1021/acscatal.5b02805). Aldolases catalyze a condensation reaction between two carbonyl compounds to form a β-hydroxy carbonyl product. Wolf-Dieter Fessner of the Technical University of Darmstadt, Pere Clapés of the Institute of Advanced Chemistry of Catalonia, and colleagues first identified key amino acid residues that could be altered to enlarge the enzyme’s binding pocket. They then used engineered E. coli to produce a range of mutant enzymes and tested them with various carbonyl substrates. The most successful mutant had two alanines in place of two leucines, which expanded the pocket to fit substrates containing up to seven carbon atoms, compared with only three for the original enzyme. Reactions pairing 3-hydroxypropanal with various hydroxyketones gave products with yields up to 89% with high stereoselectivity.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter