Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Zapping Diels-Alder reactions
Electric field spurs reagents to join up in a non-redox transformation
by Bethany Halford
March 2, 2016
| A version of this story appeared in
Volume 94, Issue 10

In a discovery that might come as a shock—or, at the very least, an electric shock—chemists have found that a properly oriented external electric field can nudge two reagents to hook up with one another in a Diels-Alder reaction. The fundamental discovery expands chemists’ knowledge of how electricity can drive synthesis and catalysis (Nature 2016, DOI: 10.1038/nature16989).
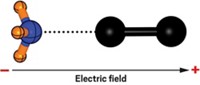
Chemists have long used electricity to trigger redox reactions. And theorists have suggested that electric fields could spur on non-redox transformations, but until now, no one had shown this was possible with a bimolecular system. “What is particularly striking is that we chose a really simple nonpolar carbon-carbon-bond-forming reaction—a Diels-Alder reaction—for which there are no formal zwitterionic intermediates involved,” says Michelle L. Coote, a professor at Australian National University who coauthored the study. “So we think these electric field effects could be very general.”
Coote and her collaborators, University of Wollongong’s Simone Ciampi and University of Barcelona’s Nadim Darwish and Ismael Diez-Perez, were inspired by the work of Hebrew University of Jerusalem theorist Sason Shaik, who suggested that an oriented electric field might accelerate Diels-Alder reactions. To prove this experimentally, Coote and her colleagues tethered a dienophile to a gold surface and a diene to the tip of a scanning-tunneling microscope.
They brought the molecules close to one another and applied an electric field. Upping the power of the electric field led to an increase in the reaction rate. What’s more, the field’s polarity mattered: The reaction rate only got a boost when the electric field favored electron flow from the dienophile to the diene.
Shaik tells C&EN he was delighted to see his theory, which had been described as “daydreaming,” proven experimentally. Although the current method can’t synthesize products on a practical scale, he thinks the discovery has the potential to change how chemists make molecules. “I definitely see a future where instead of mixing chemicals in a flask and heating, you will zap molecules with an electric field,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter