Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Bioprinted placenta model could point to preeclampsia treatments
3-D printed cell array could allow scientists to study the role of trophoblast migration in preeclampsia
by Melissa Pandika
June 14, 2016

As many as one in 10 pregnant women develop preeclampsia, a condition that can result in dangerously high blood pressure and other serious, potentially deadly complications for mother and child. Now, researchers have developed a quarter-sized, three-dimensional bioprinted model of the placenta that they hope will help better understand how preeclampsia arises and possibly lead to new treatments (ACS Biomater. Sci. Eng. 2016, DOI: 10.1021/acsbiomaterials.6b00031).
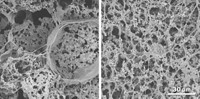
Scientists trace preeclampsia’s origins to the placenta, the organ that nourishes the developing fetus. But it is difficult to study because the placentas of common laboratory animals differ vastly from those of humans, and current 2-D in vitro models don’t reflect the complex 3-D placental environment. John P. Fisher of the University of Maryland and colleagues set out to more accurately capture this complexity by engineering the first 3-D bioprinted model of a placenta that replicates the organ’s stiffness and signaling protein gradient.
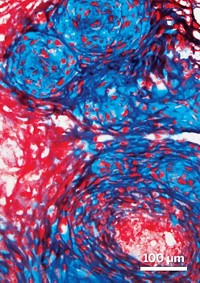
After cells called trophoblasts fasten the embryo to the uterus, they invade spiral-shaped arteries in the uterine lining, drawn by protein signals, where they begin to form the placenta. If the trophoblasts don’t invade deeply enough, the placenta can’t deliver sufficient oxygen and nutrients from the spiral arteries to the fetus, which can lead to preeclampsia.
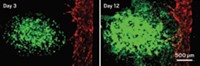
To model this critical step, the researchers decided to 3-D print a 1-mm-tall, 5-mm-wide disc out of a gelatin methacrylate (GelMA) hydrogel, with trophoblasts added along the periphery. They adjusted the percentage of polymer in the hydrogel to mimic the stiffness of human placental tissue, since that can affect cell migration. To replicate a spiral artery secreting a signaling protein, they printed a spot of epidermal growth factor (EGF), one of the protein signals known to be involved in this step, in the center.
Next, after 2-D experiments showed that trophoblasts move faster toward higher concentrations of EGF on a microscope slide, the researchers studied the effect of EGF concentration in the 3-D system. Both trophoblasts and mesenchymal stem cells, which also make up the placenta, migrated faster toward higher EGF concentrations. Their findings suggest that EGF might offer a treatment for pre-eclampsia by encouraging trophoblast penetration into the uterine lining.
But the model is only a first step. “It’s a very basic 3-D model,” says John V. Ilekis of the National Institute of Child Health & Human Development. Among the limitations, he says, are that the work used tumor-derived trophoblasts, which might behave differently from normal trophoblasts. And stimulating trophoblast invasion with EGF might not prevent preeclampsia, which may instead arise from insufficient remodeling of the spiral arteries, another part of placenta formation. Still, “this technique does have a lot of promise.”
In future work, the team plans to include more cell types. They have also begun to engineer a spiral channel in the center of the disc to better replicate a spiral artery. Eventually, the model could act as a drug discovery platform to explore how different agents might affect the placenta, says study coauthor Che-Ying Kuo of the University of Maryland.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter